Everolimus with Reduced-Dose Calcineurin Inhibitor versus Mycophenolate with Standard-Dose Calcineurin Inhibitor in De Novo Kidney Transplant Recipients: Renal Function Results from the TRANSFORM Study
1TRANSFORM Study Group, Barcelona, Spain
2Novartis Pharma AG, Basel, Switzerland.
Meeting: 2018 American Transplant Congress
Abstract number: 33
Keywords: Kidney transplantation, Multicenter studies, Proteinuria, Renal function
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: mTORi Based Regimens
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:30pm-2:42pm
Presentation Time: 2:30pm-2:42pm
Location: Room 6A
Purpose: TRANSFORM (NCT01950819) is the largest study conducted in de novo kidney transplant recipients (KTxRs) to evaluate the benefit of everolimus with reduced-dose calcineurin inhibitor (EVR+rCNI) compared to mycophenolate with standard-dose CNI (MPA+sCNI). Here, we present renal function outcomes.
Methods: In this 24-month (M), phase IV, multicenter, open-label study, adult KTxRs were randomized within 24 h of Tx to either EVR+rCNI (N=1022) or MPA+sCNI (N=1015) with induction (basiliximab or rabbit antithymocyte globulin) and steroids. Renal function assessments included eGFR (4-Variable Modification of Diet in Renal Disease) in intent-to-treat (ITT) and on-treatment populations and by donor and CNI types.
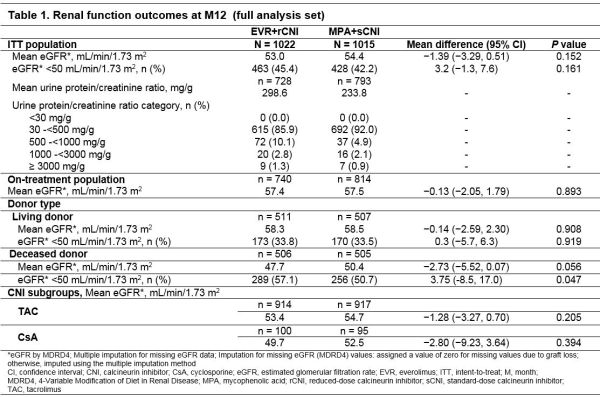
Results: Of 2037 patients, 1847 (90.7%) completed 12M study. Baseline characteristics were balanced between arms. At M12, 86.3% of patients in EVR+rCNI arm were within target EVR C0. At M12, 40.0% (TAC) and 47.6% (CsA) of patients were above target CNI C0 in EVR+rCNI arm, whereas 62.1% (TAC) and 71.6% (CsA) of patients had CNI C0 within target range in MPA+sCNI arm. Mean eGFR at M12 was comparable in EVR+rCNI vs MPA+sCNI arms in ITT (53.0 vs 54.4mL/min/1.73 m2) and on-treatment (57.4 vs 57.5mL/min/1.73 m2) patients (Table 1). Incidence of eGFR <50 mL/min/1.73 m2 was also comparable in EVR+rCNI vs MPA+sCNI arms (45.4% vs 42.2%). Mean urinary protein/creatinine ratio was 298.56 (EVR+rCNI) vs 233.8 mg/g (MPA+sCNI). Most patients (>85%) in both arms had mild proteinuria (30-<500 mg/g). With regard to donor type, eGFR was similar in EVR+rCNI and MPA+sCNI arms among patients receiving allograft from living (58.3 vs 58.5mL/min/1.73 m2) and deceased (47.7 vs 50.4mL/min/1.73 m2) donors. Mean eGFR was similar among subgroups receiving TAC and CsA in both arms.
Conclusion: In this largest study involving KTxRs, renal function outcomes were comparable between EVR-based and sCNI-based regimens in ITT population up to M12, regardless of donor and CNI types.
CITATION INFORMATION: Oppenheimer F., Legendre C., Cruzado J., Russ G., Viklicky O., Oberbauer R., Garcia V., Witzke O., Kuypers D., Danguilan R., Bernhardt P., Sommerer C. Everolimus with Reduced-Dose Calcineurin Inhibitor versus Mycophenolate with Standard-Dose Calcineurin Inhibitor in De Novo Kidney Transplant Recipients: Renal Function Results from the TRANSFORM Study Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Oppenheimer F, Legendre C, Cruzado J, Russ G, Viklicky O, Oberbauer R, Garcia V, Witzke O, Kuypers D, Danguilan R, Bernhardt P, Sommerer C. Everolimus with Reduced-Dose Calcineurin Inhibitor versus Mycophenolate with Standard-Dose Calcineurin Inhibitor in De Novo Kidney Transplant Recipients: Renal Function Results from the TRANSFORM Study [abstract]. https://atcmeetingabstracts.com/abstract/everolimus-with-reduced-dose-calcineurin-inhibitor-versus-mycophenolate-with-standard-dose-calcineurin-inhibitor-in-de-novo-kidney-transplant-recipients-renal-function-results-from-the-transform-stud/. Accessed February 23, 2026.« Back to 2018 American Transplant Congress