Two Years of the HOPE Act
1United Network for Organ Sharing, Richmond, VA
2Johns Hopkins University, Baltimore, MD.
Meeting: 2018 American Transplant Congress
Abstract number: 31
Keywords: HIV virus, Kidney transplantation, Liver transplantation
Session Information
Session Name: Concurrent Session: Kidney Deceased Donor Allocation - 1
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:30pm-3:42pm
Presentation Time: 3:30pm-3:42pm
Location: Room 6E
The HOPE Act allows research on transplantation of organs from HIV+ donors into patients infected with HIV prior to transplantation. There are 42 kidney and liver programs (37 deceased, 5 living) among 23 centers participating. Data are as of 11/24/17.
Since 1/1/16, 21 potential HIV+ deceased donors were recovered (median KDPI 40%); 19 had at least one kidney and 12 had a liver recovered for transplant. Ten (48%) were PHS Increased Risk; 3 were co-infected with HCV and/or 2 with HBV. The majority of donors were: male (76%), White (48%) or Black (43%), blood type O (52%), and had a cause of death of anoxia (43%).
Of these potential donors, 17 had organs recovered and transplanted; some could represent false positive HIV donors but that is not ascertainable with OPTN data alone. Additional confirmatory tests beyond those used for allocation suggest that 40-50% of donors were HIV false positive.
There are 198 waitlist registrations at 18 centers; 179 waiting for a kidney (86% active) and 19 waiting for a liver (79% active). One hundred and six registrations were from the waitlist that were ever willing to accept an HIV+ organ, most of which were removed for transplant (42% non-HOPE, 36% HOPE, 6% LD).
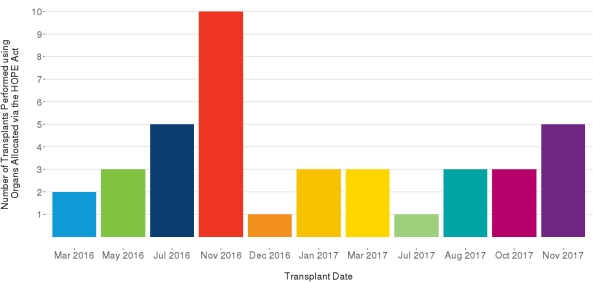
There have been 39 organ transplants performed at 6 centers (27 kidney, 12 liver; 1 en bloc kidney, 1 SLK); six occurred in the donor DSA. For kidney recipients, the median age was 53, and the majority were: male (67%), Black (74%), blood type O (52%), and not co-infected with HCV (78%) but were HBV carriers (70%). For liver recipients, the median age was 52, and the majority were: male (92%), White (42%), blood type A or O (42% each), and HCV (67%) and/or HBV carriers (50%).
Due to limited outcomes data, safety conclusions cannot be reached at this time. There has been 1 patient death and 1 graft failure reported to the OPTN. The HOPE Act continues to be underutilized.
CITATION INFORMATION: Wilk A., Durand C., Segev D., Klassen D. Two Years of the HOPE Act Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Wilk A, Durand C, Segev D, Klassen D. Two Years of the HOPE Act [abstract]. https://atcmeetingabstracts.com/abstract/two-years-of-the-hope-act/. Accessed March 8, 2026.« Back to 2018 American Transplant Congress