Evaluation of Renal Function After a Transition to Everolimus or Mycophenolate Mofetil in Liver Transplant Recipients.
1Pharmacy, Medical University of South Carolina, Chareleston, SC
2Surgery, Medical University of South Carolina, Charleston, SC
Meeting: 2017 American Transplant Congress
Abstract number: D200
Keywords: Immunosuppression, Liver transplantation
Session Information
Session Name: Poster Session D: Liver: Immunosuppression and Rejection
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Purpose: The aim of this study was to assess if there is a difference in renal function over time when transitioning to everolimus vs. mycophenolate.
Methods: This was a retrospective cohort study of adult OLTx patients between Jan 2010 through Dec 2015. All patients who were transitioned to either everolimus or mycophenolate from CNI-based regimen by post-op day 90 as CNI minimization or withdrawal were included. Multiorgan transplant patients were excluded. The primary outcome was renal function at 2-year post-transplant using MDRD. Other outcomes included discontinuation of therapy, rejection, complications, reoperations, infections, HCV recurrence, malignancy, disease recurrence, retransplant, lab findings, graft loss, and death.
Results: A total of 96 patients were included. Baseline characteristics were similar between groups, with differences noted in primary insurance, presence of HCC, NASH as etiology of ESLD, functional status, and MELD score (Table 1). Based on the change in eGFR slope overtime, there was a trend towards worsening renal function in the everolimus group at 1-year post-transplant (Table 2 & Figure 1, p=0.081). 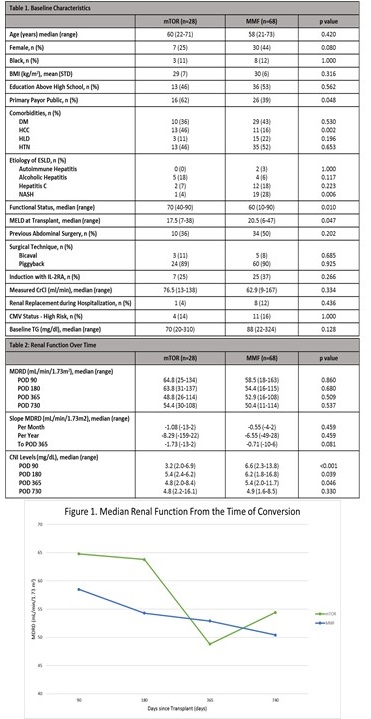 Discontinuation of therapy occurred in 15 (54%) patients in the everolimus group versus 26 (38%) in the mycophenolate group (p=0.167). There was no difference between groups in other clinical outcomes (Table 3, Table 4 & Figure 2).
Discontinuation of therapy occurred in 15 (54%) patients in the everolimus group versus 26 (38%) in the mycophenolate group (p=0.167). There was no difference between groups in other clinical outcomes (Table 3, Table 4 & Figure 2). 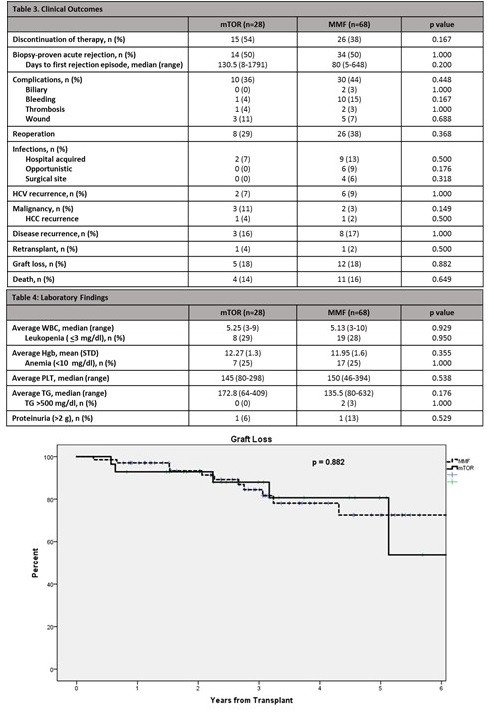 Conclusion: This data demonstrates that the adjunctant use of everolimus, as compared to mycophenolate in liver transplant recipients, produced a trend toward worsening renal function and higher discontinuation rates with similar clinical outcomes.
Conclusion: This data demonstrates that the adjunctant use of everolimus, as compared to mycophenolate in liver transplant recipients, produced a trend toward worsening renal function and higher discontinuation rates with similar clinical outcomes.
CITATION INFORMATION: Perez C, Fleming J, Sell M, O'brien B, Rogers A, Lee I, Mardis C, Mardis A, Patel N, Meadows H, Pilch N, Chavin K, Dubay D, Vinayak R, Taber D. Evaluation of Renal Function After a Transition to Everolimus or Mycophenolate Mofetil in Liver Transplant Recipients. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Perez C, Fleming J, Sell M, O'brien B, Rogers A, Lee I, Mardis C, Mardis A, Patel N, Meadows H, Pilch N, Chavin K, Dubay D, Vinayak R, Taber D. Evaluation of Renal Function After a Transition to Everolimus or Mycophenolate Mofetil in Liver Transplant Recipients. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluation-of-renal-function-after-a-transition-to-everolimus-or-mycophenolate-mofetil-in-liver-transplant-recipients/. Accessed February 22, 2026.« Back to 2017 American Transplant Congress
