Risks Associated with Recombinant IL2 Therapy in Renal Transplantation.
Brigham and Women's Hospital, Boston
Meeting: 2017 American Transplant Congress
Abstract number: D112
Keywords: Immunosuppression, Kidney transplantation, Lymphocytes
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Regulatory T cells are critical to allograft survival. We report a pilot study of safety and tolerability of low dose recombinant IL2 (rIL2), 1×106 units/m2/day SQ in renal transplant (RTx) recipients, to promote Tregs in vivo.
Inclusion criteria: >6 months post RTx; biopsy showing interstitial fibrosis and tubular atrophy (IFTA) ≥ grade 2 with lymphocytic infiltration, without rejection. Treatment was for 6 weeks, with optional extension phase. Two patients have enrolled.
Patient 1 received RTx in 2014. Creatinine (Cr) was 1.4mg/dL and biopsy showed grade 2-3 IFTA with chronic inflammation. On day 9 of rIL2 2.27×106 IU, he reported myalgia, sinus symptoms and low-grade fever. On day 14, Cr was 1.96mg/dL. Biopsy showed Banff grade 1a acute cellular rejection. After pulse steroids, Cr returned to baseline.
Flow cytometry of PBMCs showed no increase in Tregs in response to rIL2, with CD4+CD25+CD127- cells 1.24% at week 0, falling to 0.76% and 0.84% at week 1 and 2 respectively. In contrast, both CD16-CD56+ and CD16+CD56+ NK cells increased. (CD16-CD56+ 1.58%, 2.4%, 5.64%; CD16+CD56+ 3.51%, 1.99% and 13.1% at 0, 1 and 2 weeks) 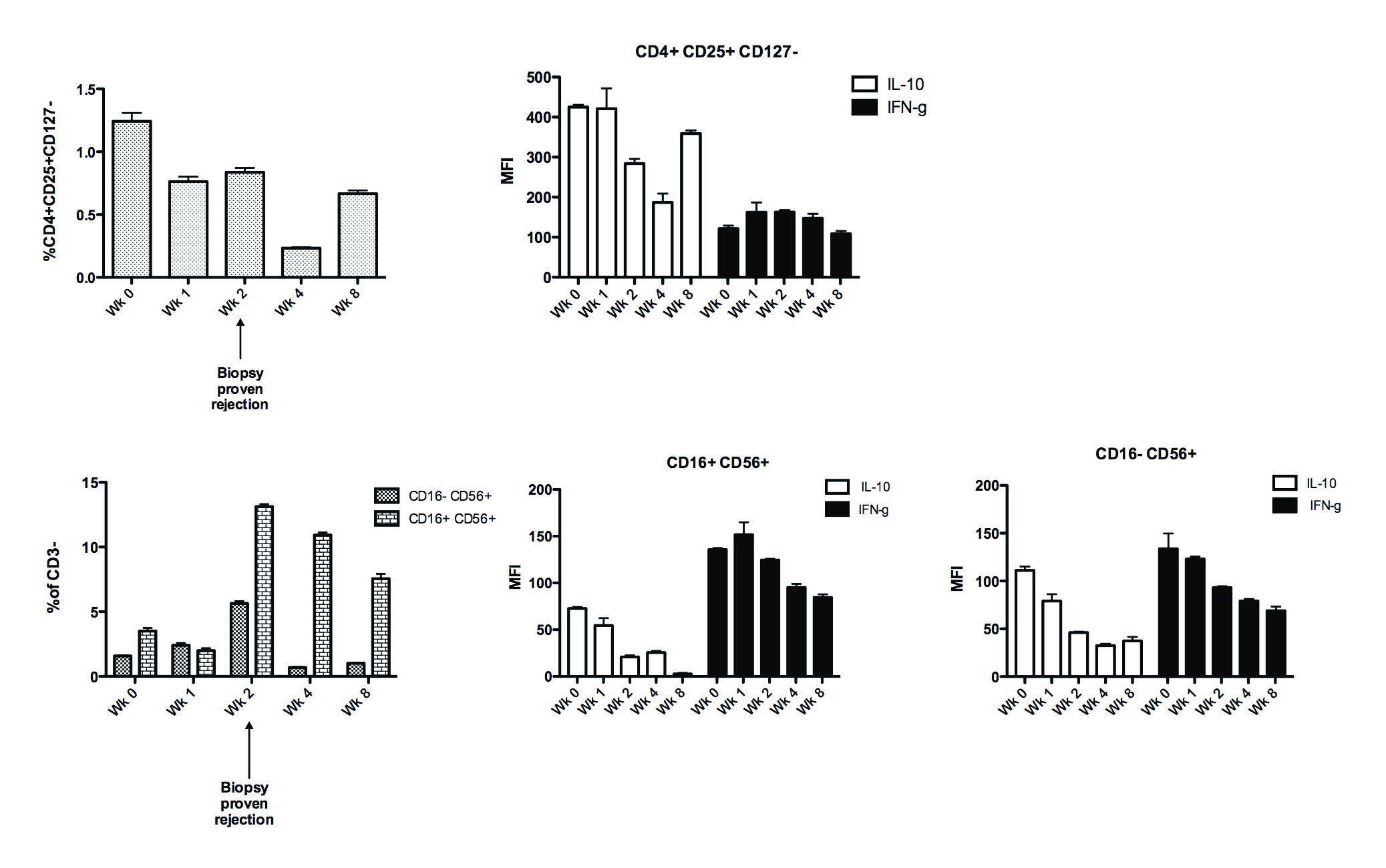 Treg and NK cytokine production was unchanged. Renal biopsy is being stained for CD3, Foxp3 and CD56.
Treg and NK cytokine production was unchanged. Renal biopsy is being stained for CD3, Foxp3 and CD56.
Patient 2 received DCD RTx in 2005. Biopsy showed severe transplant glomerulopathy, grade 2 IFTA and interstitial inflammation. Cr was 3mg/dL. After 6 weeks of rIL2 2.05×106 IU, he had stable function. At week 10, he became unwell and after a complex course, he initiated chronic hemodialysis.
PBMC analysis showed a >8 fold rise in Tregs at week 1 (4.44% to 37.96%, P<0.001) which was sustained. Decreased CD16-CD56+ and increased CD16+CD56+ NK were seen, with markedly reduced IFNg production.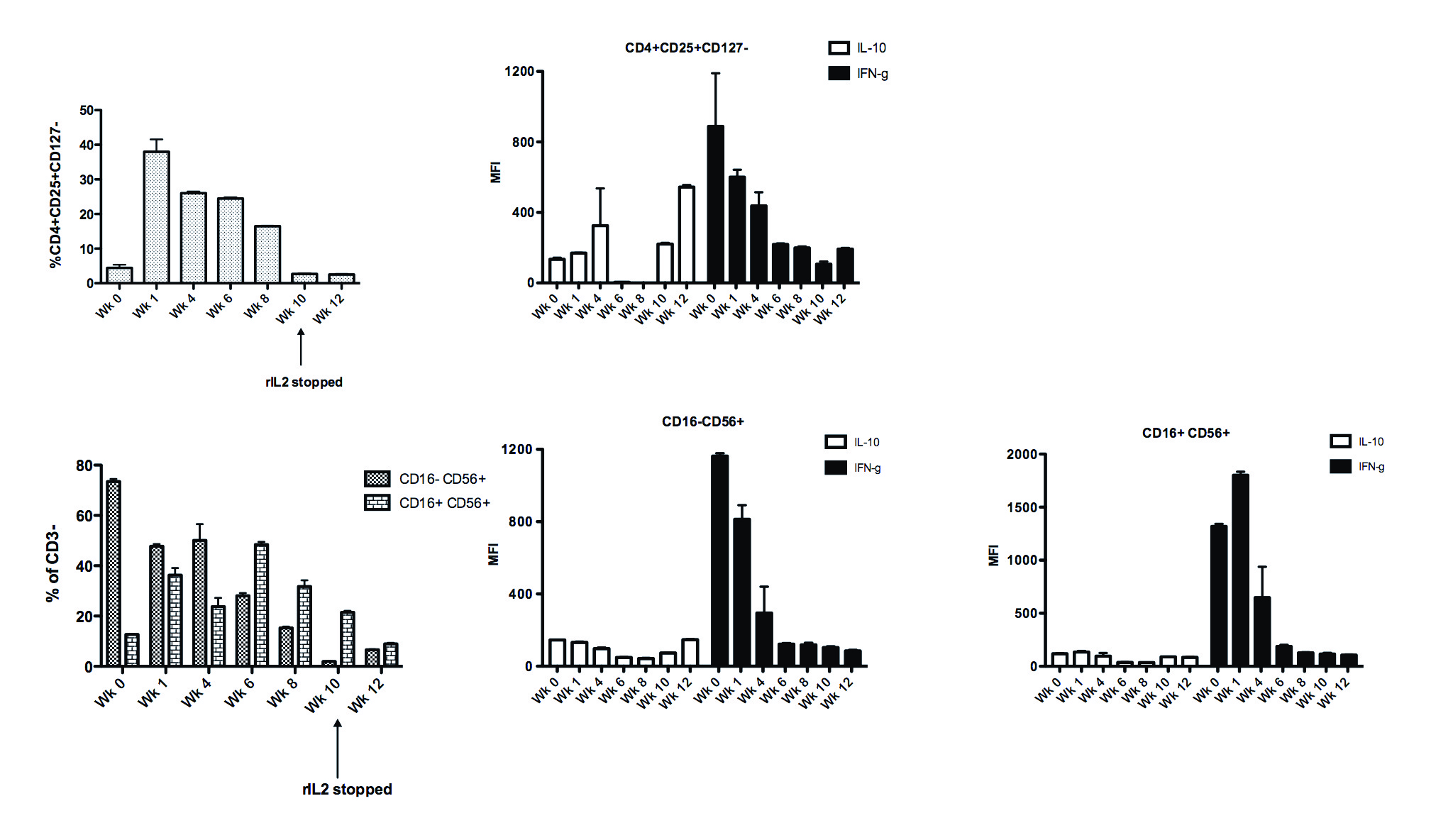 Our findings show that rIL2 can lead to variable changes in Treg and NK cells in transplant recipients and illustrate the challenges of this approach in medically complex patients.
Our findings show that rIL2 can lead to variable changes in Treg and NK cells in transplant recipients and illustrate the challenges of this approach in medically complex patients.
CITATION INFORMATION: McGrath M, Tripathi S, Chandraker A. Risks Associated with Recombinant IL2 Therapy in Renal Transplantation. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
McGrath M, Tripathi S, Chandraker A. Risks Associated with Recombinant IL2 Therapy in Renal Transplantation. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/risks-associated-with-recombinant-il2-therapy-in-renal-transplantation/. Accessed January 14, 2026.« Back to 2017 American Transplant Congress
