Alemtuzumab Induction and Belatacept Maintenance in Marginal Pathology Renal Allografts.
University of Maryland, Baltimore
Meeting: 2017 American Transplant Congress
Abstract number: D80
Keywords: Co-stimulation, Induction therapy, Kidney transplantation
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Background: The potential for improved outcomes with CNI avoidance may be important for kidneys with an increased likelihood of impaired graft function. We performed a pilot study investigating the use of alemtuzumab induction and belatacept maintenance therapy in renal allografts with pre-existing pathology.
Methods: A prospective, IRB-approved pilot study of adult renal transplant recipients receiving a primary deceased donor renal allograft. Patients had an intermediate Maryland Aggregate Pathology Index (MAPI) score, which was used to identify kidneys at increased risk for graft failure. Study recipients received alemtuzumab induction, belatacept dosed per product labeling, MMF 2g/day, and a 21-day steroid withdrawal taper. Study participants were retrospectively matched 1:1 on the basis of MAPI score, age, gender, and race to patients who received tacrolimus. The primary endpoint was eGFR at 3 and 12 months post-transplant, with secondary endpoints of biopsy-proven rejection, patient survival, and graft survival at 12 months.
Results: Select demographics and outcomes are shown in the table. While not significantly different between groups, eGFR was higher in belatacept recipients at both 3 and 12 months post-transplant. Graft failure by 12 months occurred in 1 patient in the belatacept group due to rejection related to non-compliance and 2 patients in the tacrolimus group due to graft infarction approximately 5 months post-transplant and primary non-function, respectively. Rejection-free graft survival was not significantly different between groups (p=0.6). One year patient survival was comparable between groups.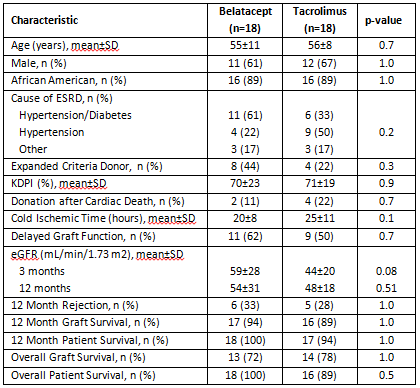 Conclusions: Alemtuzumab induction mitigates higher rejection rates seen in previous studies utilizing belatacept-based immunosuppressive platforms, while allowing for improved longer term function. These data support tailored immunosuppressive strategies to improve outcomes of kidneys at higher risk for poor function and outcomes.
Conclusions: Alemtuzumab induction mitigates higher rejection rates seen in previous studies utilizing belatacept-based immunosuppressive platforms, while allowing for improved longer term function. These data support tailored immunosuppressive strategies to improve outcomes of kidneys at higher risk for poor function and outcomes.
CITATION INFORMATION: Sparkes T, Ravichandran B, Opara O, Ugarte R, Bromberg J, Barth R. Alemtuzumab Induction and Belatacept Maintenance in Marginal Pathology Renal Allografts. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Sparkes T, Ravichandran B, Opara O, Ugarte R, Bromberg J, Barth R. Alemtuzumab Induction and Belatacept Maintenance in Marginal Pathology Renal Allografts. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/alemtuzumab-induction-and-belatacept-maintenance-in-marginal-pathology-renal-allografts/. Accessed February 10, 2026.« Back to 2017 American Transplant Congress
