Prevalence of CYP3A Genomic Variances and Impact on Tacrolimus Dosing Requirements in Kidney Transplant Recipients in Eastern North Carolina.
1Vidant Medical Center, Greenville, NC
2East Carolina University, Greenville, NC
Meeting: 2017 American Transplant Congress
Abstract number: D75
Keywords: African-American, Dosage, Immunosuppression, Kidney transplantation
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
BACKGROUND: African American (AA) patients are substantially more likely to express CYP3A5. Presence of CYP3A5 polymorphisms is an important pharmacotherapeutic consideration due to the extensive CYP metabolism of tacrolimus (TAC). We aim to assess the role of CYP3A5 genomic variances and impact on TAC dosing in a program with a high prevalence of potential CYP3A5*1 expressers.
METHODS: Retrospective longitudinal cohort study at a large tertiary medical center. 162 kidney transplant recipients (KTR) were included in the study, comprising CYP3A5*1 expressers (n=85; 52%) vs non-expressers (n=77; 48%). Primary endpoint = TAC total daily dose (TDD) to achieve a therapeutic trough based on the presence of the CYP3A5*1 variant. Secondary endpoint = prevalence of CYP3A5 variants across races.
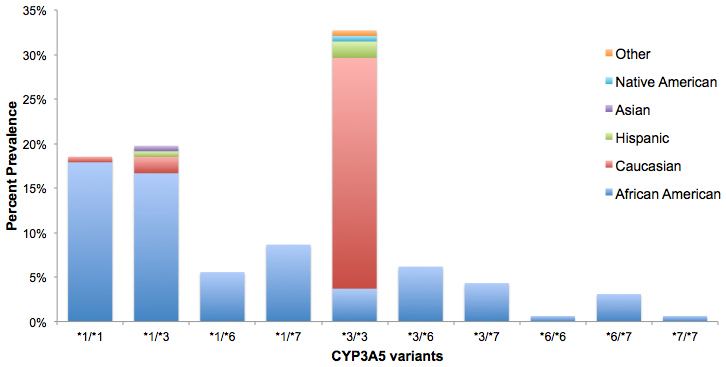
RESULTS: By multivariate analysis, CYP3A5*1 expressers and non-expressers did not differ significantly with respect to gender, or mean age. The frequency of any CYP3A5*1 expression within the cohort was 52%, and largely AA (93%; p<0.0005). Among CYP3A5*1 expressers compared to non-expressers, mean TDD at first therapeutic level was significantly higher (12mg/day vs. 8mg/day; p≤0.001). Mean mg/kg TDD was 50% greater among CYP3A5*1 expressers (0.15mg/kg/day vs. 0.1mg/kg/day; p≤0.0005). Notable genotypic frequencies were CYP3A5*3/*3 (32%), CYP3A5*1/*3 (20%) and CYP3A5*1/*1 (19%).
CONCLUSION: This single-center study confirms the prevalence of the CYP3A5*1 gene amongst AA KTR and the effect of this gene expression on the dosing of TAC. The CYP3A5*1 gene influences TDD and these patients require higher TAC doses to achieve desirable drug levels. AA KTR who express CYP3A5*1 may be at higher risk for poor clinical outcomes related to inadequate immunosuppression. 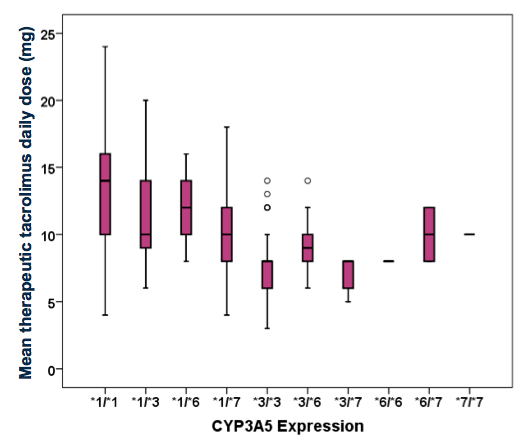
CITATION INFORMATION: Asempa T, Hudson S, Rebellato L, Maldonado A. Prevalence of CYP3A Genomic Variances and Impact on Tacrolimus Dosing Requirements in Kidney Transplant Recipients in Eastern North Carolina. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Asempa T, Hudson S, Rebellato L, Maldonado A. Prevalence of CYP3A Genomic Variances and Impact on Tacrolimus Dosing Requirements in Kidney Transplant Recipients in Eastern North Carolina. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/prevalence-of-cyp3a-genomic-variances-and-impact-on-tacrolimus-dosing-requirements-in-kidney-transplant-recipients-in-eastern-north-carolina/. Accessed February 11, 2026.« Back to 2017 American Transplant Congress
