TNF[part]-I After Kidney Transplantation.
Department of Medicine, Division of Nephrology, University of Wisconsin, Madison, WI
Meeting: 2017 American Transplant Congress
Abstract number: C165
Keywords: Infection, Kidney transplantation, Tumor necrosis factor (TNF)
Session Information
Session Name: Poster Session C: Kidney Complications III
Session Type: Poster Session
Date: Monday, May 1, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Infliximab, adalimumab and etanercept are monoclonal antibodies which inhibit tumor necrosis factor alpha (TNF[part]-I) and are used in the treatment of inflammatory diseases such as rheumatoid arthritis (RA) and inflammatory bowel disease (IBD). TNF[part]-I are associated with an increased risk of infections and malignancies. There is no data on outcomes in kidney transplant recipients (KTR) who receive TNF[part]-I.
Here we used the Wisconsin Allograft Recipient Database (WisARD) to describe 15 KTR who received TNF[part]-I between 1997 and 2013. Six patients who received TNF[part]-I pre-transplant were excluded from analysis, as they did not continue therapy post-transplant. Five of the 9 remaining patients were diagnosed with inflammatory disease pretransplant, 4 were diagnosed posttransplant. 1 patient was treated with infliximab for IBD during 2 KTs, 1 patient was treated with adalimumab, then etanercept for RA.
All patients were Caucasian. All were on triple immunosuppressive therapy at transplant except patient #7 who was on dual therapy after early steroid withdrawal. Baseline characteristics are in table 1. 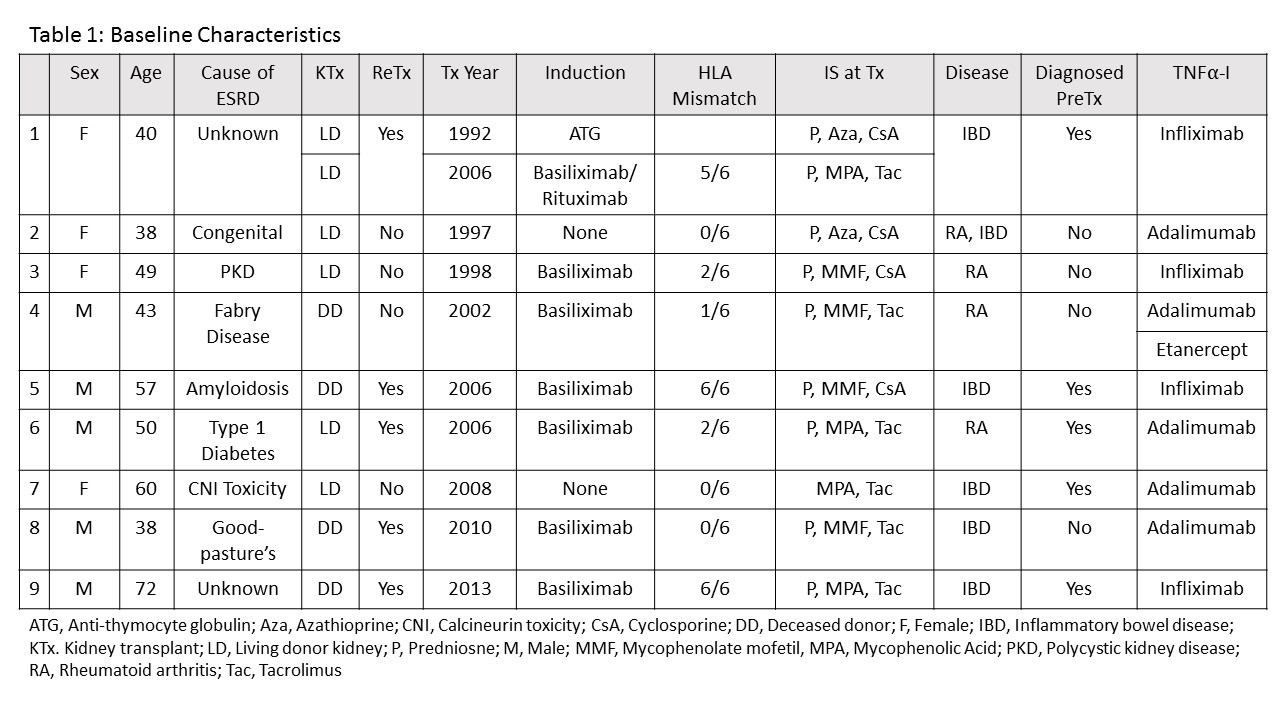 Two months after starting TNF[part]-I, the previous immunosuppression (IS) was not changed in 7 patients. The antimetabolite was stopped in 2, SRL was added in 1 and prednisone was decreased in 1. Eight patients had at least 1 infection while receiving TNF[part]-I. Patient #4 had significant infectious complications and died of sepsis. The antimetabolite was stopped in patient #8, this patient had no infections. 3 patients had skin cancers, 1 had lung cancer. Patients #1 and 7 had an episode of rejection. Outcomes are in table 2.
Two months after starting TNF[part]-I, the previous immunosuppression (IS) was not changed in 7 patients. The antimetabolite was stopped in 2, SRL was added in 1 and prednisone was decreased in 1. Eight patients had at least 1 infection while receiving TNF[part]-I. Patient #4 had significant infectious complications and died of sepsis. The antimetabolite was stopped in patient #8, this patient had no infections. 3 patients had skin cancers, 1 had lung cancer. Patients #1 and 7 had an episode of rejection. Outcomes are in table 2. 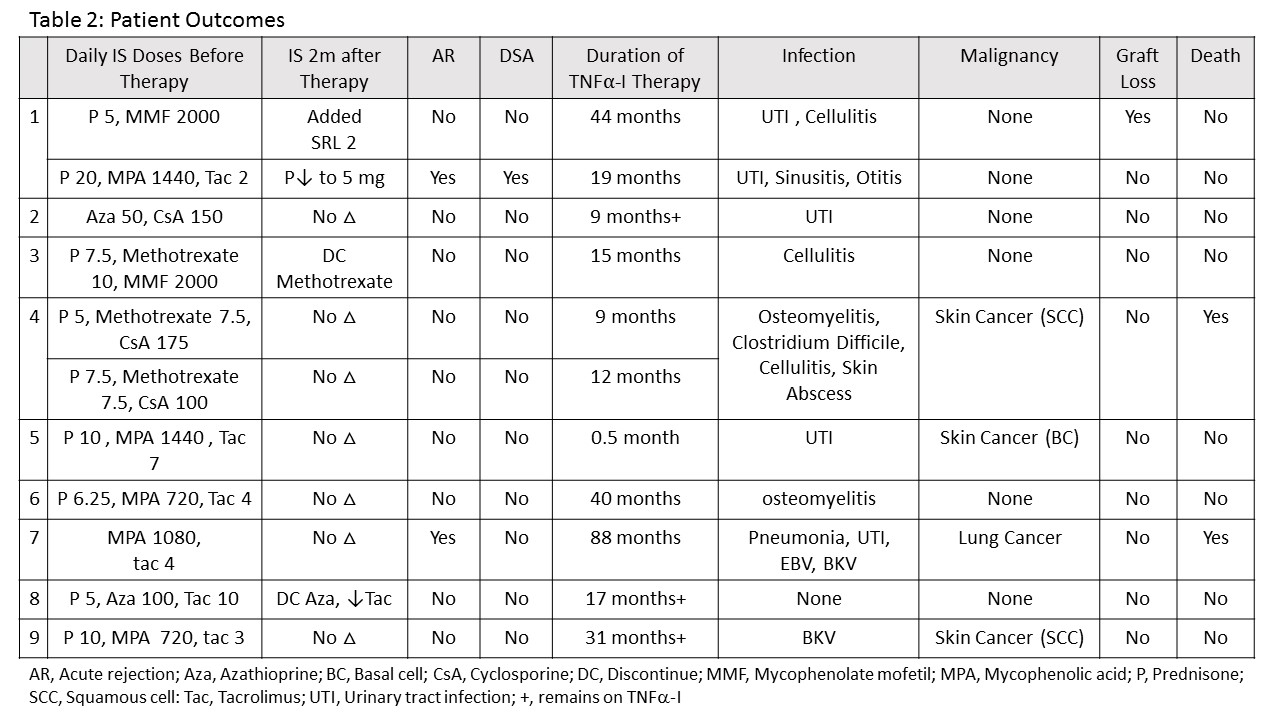 Infections and malignancies are known complications of both transplant IS and TNF[part]-I. TNF[part]-I can be used in KT, but the rate of infection is high. We suggest that KTR who require treatment with TNF[part]-I have their baseline IS decreased, for example by stopping or reducing the antimetabolite.
Infections and malignancies are known complications of both transplant IS and TNF[part]-I. TNF[part]-I can be used in KT, but the rate of infection is high. We suggest that KTR who require treatment with TNF[part]-I have their baseline IS decreased, for example by stopping or reducing the antimetabolite.
CITATION INFORMATION: Muth B, Astor B, Mandelbrot D. TNF[part]-I After Kidney Transplantation. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Muth B, Astor B, Mandelbrot D. TNF[part]-I After Kidney Transplantation. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/tnfpart-i-after-kidney-transplantation/. Accessed February 24, 2026.« Back to 2017 American Transplant Congress
