National Variation in US Kidney Transplant Induction Therapy.
1Washington Univ, St. Louis, MO
2U Michigan, Ann Arbor, MI
3East Carolina Univ, Greenville, NC
4St. Louis Univ, St. Louis, MO
5Johns Hopkins, Baltimore, MD
6Scientific Registry of Transplant Recipients, Minneapolis, MN
Meeting: 2017 American Transplant Congress
Abstract number: B182
Keywords: Induction therapy, Kidney transplantation, Multivariate analysis, Resource utilization
Session Information
Session Name: Poster Session B: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Sunday, April 30, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Antibody induction (IND) therapy in kidney transplantation (KTx) reduces acute rejection risk but adds to transplant cost. Currently, only IL2 Receptor antibodies (IL2R-Ab) have FDA approval for IND, but thymoglobulin (TMG), alemtuzumab (ALEM), and other agents are often used.
To quantify the impact of program and case factors in IND regimen choice in US practice, we examined national transplant registry data for 166,776 KTx recipients (2005-2015). Bi-level hierarchical models were constructed; use of each regimen was compared to IL2R-Ab (pairwise). Metrics of heterogeneity included intraclass correlation (ICC), the ratio of cluster variance (program impact) to total observed variance in IND use. Median odds ratios (MOR) provided the median odds that patients with identical characteristics receive the regimen when 2 programs are randomly drawn.
Overall, IND was most commonly administered as TMG (46.0%), followed by IL2R-Ab (22.0%), ALEM (12.5%), and Other (1.3%); no IND was reported for 18.2%. Choice of IND varied widely (0% to 100% of KTx) across programs (Fig).
After adjustment for case factors, the ICCs suggest that most variation in TMG (58%), ALEM (66%), Other (51%), and No IND (58%) use reflects “program effect.” MORs from case-factor adjusted models for each regimen compared with reference ranged from 7.66 to 11.19 (Fig).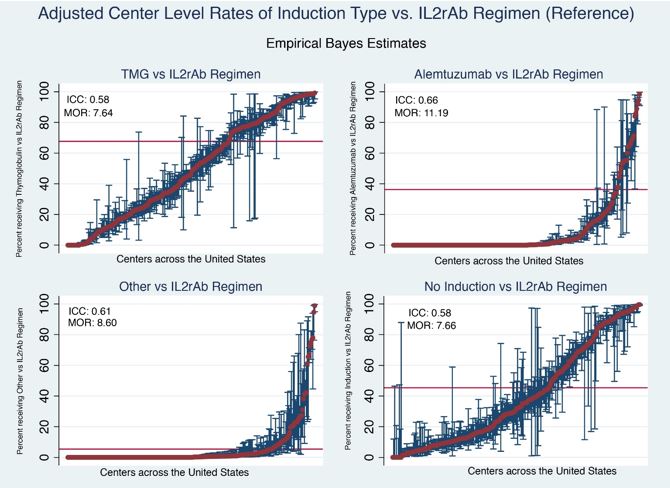 While explaining <5% variation, case factors that correlate with TMG and ALEM compared to IL2R-Ab included black race, sensitization, retransplant status, non-standard deceased donor, and delayed graft function; use was less common in the elderly. No IND was more common for living donor KTx recipients or those without Medicare or private insurance.
While explaining <5% variation, case factors that correlate with TMG and ALEM compared to IL2R-Ab included black race, sensitization, retransplant status, non-standard deceased donor, and delayed graft function; use was less common in the elderly. No IND was more common for living donor KTx recipients or those without Medicare or private insurance.
Use of KTx IND varies widely across US transplant programs; choice largely reflects program biases, not patient characteristics or evidence of comparative efficacy.
CITATION INFORMATION: Dharnidharka V, Naik A, Alhamad T, Axelrod D, Schnitzler M, Zhang Z, Bae S, Brennan D, Segev D, Randall H, Nazzal M, Ouseph R, Kasiske B, Lentine K. National Variation in US Kidney Transplant Induction Therapy. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Dharnidharka V, Naik A, Alhamad T, Axelrod D, Schnitzler M, Zhang Z, Bae S, Brennan D, Segev D, Randall H, Nazzal M, Ouseph R, Kasiske B, Lentine K. National Variation in US Kidney Transplant Induction Therapy. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/national-variation-in-us-kidney-transplant-induction-therapy/. Accessed March 4, 2026.« Back to 2017 American Transplant Congress
