A Phase Ib, Open Label, Single Arm Study to Assess the Safety, Pharmacokinetics, and Impact on Humoral Sensitization of SANGUINATE Infusion in Patients with End-Stage Renal Disease.
1U.Cincinnati, OH
2Prolong Pharmaceuticals, NJ
Meeting: 2017 American Transplant Congress
Abstract number: B160
Keywords: Alloantibodies, Kidney transplantation, Pharmacokinetics, Sensitization
Session Information
Session Name: Poster Session B: Kidney Complications II
Session Type: Poster Session
Date: Sunday, April 30, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Purpose:The endeavor and resources spent to study desensitization haven't been matched by an effort to investigate strategies to prevent sensitization.The purpose of this study was to assess the impact on sensitization, pharmacokinetics(PK), and safety of SANGUINATE (SG) as a potential alternative to PRBC transfusion in dialysis patients.SG is pegylated bovine carboxyhemoglobin that acts as an oxygen transfer agent and releases low levels of carbon monoxide which ameliorates ischemia-reperfusion injury and potentially delayed graft function.
Methods:Upon informed consent,10 ESRD subjects on chronic hemodialysis, meeting study inclusion/exclusion criteria were planned to receive 3 weekly infusions of SG (320mg/kg per infusion).Serial blood samples were obtained to determine the SG PK profile and assess any impact on anti-HLA antibodies (Abs).The subjects were followed for 90 days for safety.
Results:The sponsor stopped the study after 5 subjects were enrolled.Demographic information is provided in Table 1.Subject 5 withdrew consent on Day 8 after a SG infusion.No subject developed new Abs after SG infusion throughout the study period.SG PK analysis showed mean(sd) Cmax, Tmax, AUC and half-life of 4.6(0.8)mg/ml, 3.1(1.0)hrs, 159.4(33.8)mg.hr/ml and 27.2(9.5)hrs respectively.Safety analysis revealed Subject 3 developed an NSTEMI after his third SG infusion with peak troponin of 0.26ng/ml. A left heart cath (LHC) was performed revealing an 80% right coronary artery stenosis that was stented. Interpretation of this event was complicated by the patient's admission of cocaine use.Subject 5 had an elevated troponin which peaked at 0.58ng/ml after her first SG infusion.LHC was performed and showed normal coronaries.All observed adverse events were transient and are presented in Table 2.
Conclusion:SG is a potential alternative to PRBCs in ESRD patients considered for renal transplantation as it was not associated with humoral sensitization.Larger studies are needed to further evaluate any potential safety signals.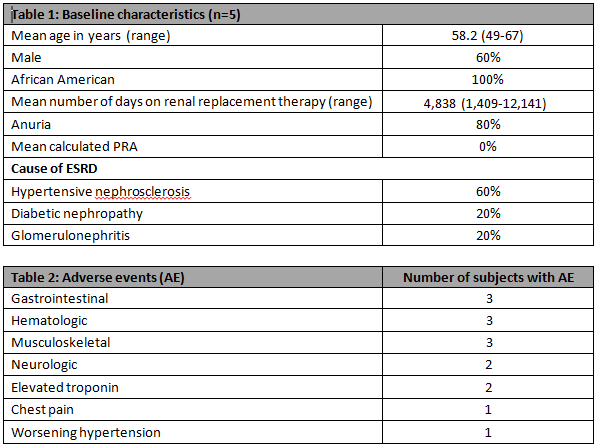
CITATION INFORMATION: Abu Jawdeh B, Woodle E, Leino A, Dorst T, Abdallah M, Govil A, Byczkowski D, Misra H, Abuchowski A, Alloway R. A Phase Ib, Open Label, Single Arm Study to Assess the Safety, Pharmacokinetics, and Impact on Humoral Sensitization of SANGUINATE Infusion in Patients with End-Stage Renal Disease. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Jawdeh BAbu, Woodle E, Leino A, Dorst T, Abdallah M, Govil A, Byczkowski D, Misra H, Abuchowski A, Alloway R. A Phase Ib, Open Label, Single Arm Study to Assess the Safety, Pharmacokinetics, and Impact on Humoral Sensitization of SANGUINATE Infusion in Patients with End-Stage Renal Disease. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/a-phase-ib-open-label-single-arm-study-to-assess-the-safety-pharmacokinetics-and-impact-on-humoral-sensitization-of-sanguinate-infusion-in-patients-with-end-stage-renal-disease/. Accessed February 16, 2026.« Back to 2017 American Transplant Congress
