Acthar in Recurrent Focal Segmental Glomerular Sclerosis After Kidney Transplantation.
1Washington University in St. Louis, St. Louis
2Johns Hopkins University, Baltimore
Meeting: 2017 American Transplant Congress
Abstract number: B150
Keywords: Glomerulonephritis, Proteinuria
Session Information
Session Name: Poster Session B: Kidney Complications II
Session Type: Poster Session
Date: Sunday, April 30, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Treatment of Recurrent focal segmental glomerular sclerosis (FSGS) after kidney transplantation is challenging with unpredictable outcomes. Conventional therapeutic options include various combinations of plasmapheresis, steroids, ACE inhibitors/ARB and rituximab. H.P. Acthar Gel (adrenocorticotropic hormone analogue injection) is FDA approved for treatment of nephrotic syndrome and its use in FSGS has been reported in a few case-reports.
We investigated the use of Acthar Gel in kidney transplant recipients with recurrent FSGS at two large transplant centers between April 2012 and June 2016. Proteinuria was measured by urine protein to creatinine ratio (UPCR). Complete remission was defined as a decrease of proteinuria by 50% and to less than 1.5 g/g. Partial remission was defined as a decrease of proteinuria by 50% and to a level between 1.5 to 3 g/g.
19 cases were identified. Mean age was 49, 21% were female, 62% Caucasians, and 31% African American. Mean time from initial native FSGS diagnosis to end stage renal disease was 3.5 years. 58% received kidney transplantation from deceased donors. 47% received rituximab as a prevention at the time of transplant or treatment for recurrence before Acthar and 68% had plasmapheresis with or before Acthar. On average, there was a significant improvement of proteinuria [from mean 11.5 (SD±8.1) to 5.3 (±2.6) g, P=0.0024], creatinine [from 4.2 (±2.4) to 2.6 (±1.9) mg/dl, P=0.042] and GFR [from 26.6 (±18) to 39.3 (±22) mL/min/1.73 m2, P=0.051]. 26% had complete remission and 21% had partial remission. 4 allografts failed during the follow-up (median 30 months), 3 were attributed to recurrent FSGS between 1 and 3 years despite the use of Acthar. 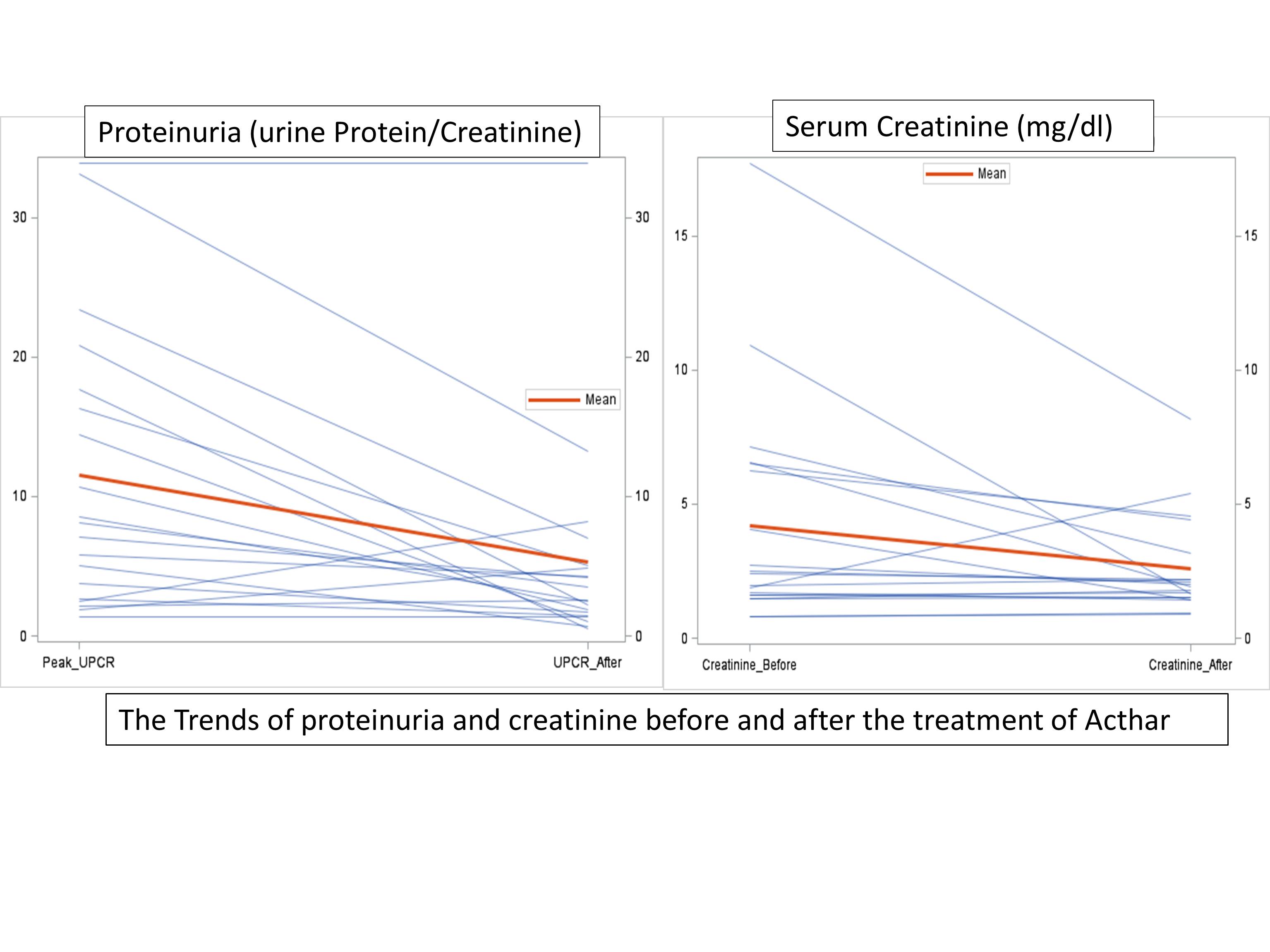 The response to Acthar varied among the recipients. Around 50% had significant reduction of proteinuria consistent with the definition of partial and complete remission. Acthar might be an effective therapy for recurrent FSGS.
The response to Acthar varied among the recipients. Around 50% had significant reduction of proteinuria consistent with the definition of partial and complete remission. Acthar might be an effective therapy for recurrent FSGS.
CITATION INFORMATION: Alhamad T, Dieck J, Vujjini V, Kanawati B, Brennan D, Alachkar N. Acthar in Recurrent Focal Segmental Glomerular Sclerosis After Kidney Transplantation. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Alhamad T, Dieck J, Vujjini V, Kanawati B, Brennan D, Alachkar N. Acthar in Recurrent Focal Segmental Glomerular Sclerosis After Kidney Transplantation. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/acthar-in-recurrent-focal-segmental-glomerular-sclerosis-after-kidney-transplantation/. Accessed February 16, 2026.« Back to 2017 American Transplant Congress
