IVIG + Rituximab as Desensitization Agents to Reduce Donor Specific Antibodies and Improve Transplant Rates in Highly Hla Sensitized (HS) Awaiting Deceased Donor Kidney Transplantation.
Medicine, Cedars-Sinai Medical Center, Los Angeles, CA
Meeting: 2017 American Transplant Congress
Abstract number: B74
Keywords: HLA antibodies
Session Information
Session Name: Poster Session B: Antibody Mediated Rejection in Kidney Transplant Recipients II
Session Type: Poster Session
Date: Sunday, April 30, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Background: HS patients are at risk for development of donor specific antibodies (DSA) and the persistence of DSA is associated with poor graft outcomes. Rituximab, an anti-CD20, is used for B-cell depletion and reduction in DSA. Here we report outcomes of a prospective clinical trial evaluating the efficacy of rituximab in lowering DSA levels and reducing allograft injury post-transplant Methods: From 2013-2016, 39 HS patients with cPRA≥50% underwent desensitization (DES) Acceptable crossmatch criteria at transplant included: a negative CDC at 1:2 dilution; T& B-cell FCMX ≤ 225MCS Unacceptable antigens were defined as MFI>15,000. Patient and graft survival, freedom from ABMR, and DSA RIS score were analyzed. Patients with persistent DSA at 3M or 6M received one additional dose of rituximab 1 gm. Fifteen of 26 patients underwent protocol biopsy at 12M. Results: Twenty-six (67%) were transplanted after rituximab + IVIG DES. Nineteen patients (73%) were DSA+ @transplant. Of these, fifteen (58%) patients were also FCMX+. Baseline mean RIS score was 3.9 (±4.5) and decreased to 1.0 ((±2.5) by 12 months (Fig. 1, p=0.002). The actuarial incidence of ABMR was 16% at 2.5 years (Fig. 2a). Of those with ABMR on for-cause biopsies, Banff severity scores were relatively mild (Fig. 2b). On protocol biopsies at 12 months, there was a paucity of ABMR lesions, with only 2 patients found to have ABMR lesions. Both were mild with negative C4d and PTC+G and TG scores of 1 (Fig. 2b). Nine patients (35%) received an additional dose of rituximab at 3M or 6M post-transplant. Patient and graft survival was 100% and 96% @ 12M. There was one graft loss day 1 post Tx was deemed technical-related. 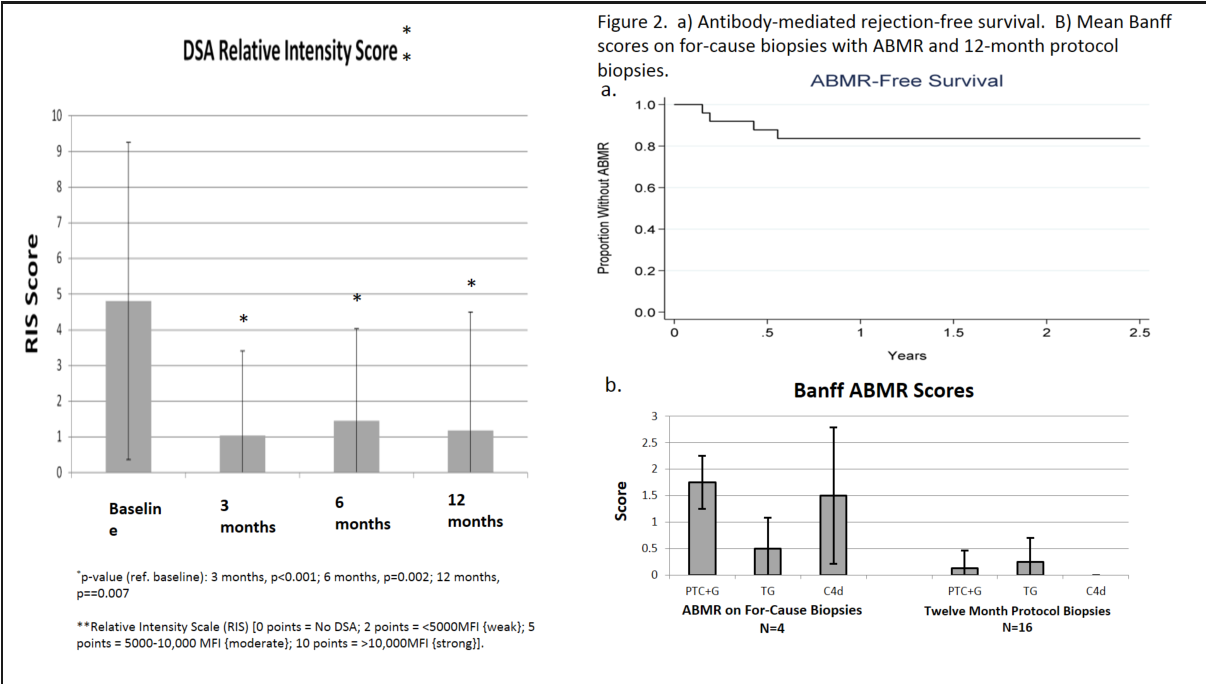 Conclusions: Rituximab + IVIG is an effective and safe desensitization protocol. The addition of rituximab appeared to be efficacious in preventing ABMR and DSA rebound. Treatment with rituximab for persistent DSA at 3 or 6M post-transplant was associated with a significant reduction in DSA by 12M with low rates of ABMR, TG and graft loss.
Conclusions: Rituximab + IVIG is an effective and safe desensitization protocol. The addition of rituximab appeared to be efficacious in preventing ABMR and DSA rebound. Treatment with rituximab for persistent DSA at 3 or 6M post-transplant was associated with a significant reduction in DSA by 12M with low rates of ABMR, TG and graft loss.
CITATION INFORMATION: Najjar R, Huang E, Vo A, Cho J, Louis S, Hang A, Peng A, Jordan S. IVIG + Rituximab as Desensitization Agents to Reduce Donor Specific Antibodies and Improve Transplant Rates in Highly Hla Sensitized (HS) Awaiting Deceased Donor Kidney Transplantation. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Najjar R, Huang E, Vo A, Cho J, Louis S, Hang A, Peng A, Jordan S. IVIG + Rituximab as Desensitization Agents to Reduce Donor Specific Antibodies and Improve Transplant Rates in Highly Hla Sensitized (HS) Awaiting Deceased Donor Kidney Transplantation. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/ivig-rituximab-as-desensitization-agents-to-reduce-donor-specific-antibodies-and-improve-transplant-rates-in-highly-hla-sensitized-hs-awaiting-deceased-donor-kidney-transplantation/. Accessed February 27, 2026.« Back to 2017 American Transplant Congress
