Impact of Filgrastim Use on Maintenance Doses of Immunosuppressants and Clinical Outcomes in Renal Transplant Patients.
1University of Kentucky HealthCare, Lexington, KY
2University of Kentucky College of Pharmacy, Lexington, KY
Meeting: 2017 American Transplant Congress
Abstract number: A204
Keywords: Kidney transplantation, Neutropenia
Session Information
Session Name: Poster Session A: Kidney Complications I
Session Type: Poster Session
Date: Saturday, April 29, 2017
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall D1
Introduction: There are limited data assessing the outcomes of filgrastim for the treatment of neutropenia among renal transplant patients. We investigated the association of filgrastim with the incidence of acute rejection and infection, changes in mycophenolate mofetil (MMF) dose, and graft and patient survival in this group.
Methods: This was a retrospective, single center matched control study of adult renal transplant patients from 2010 to present who received filgrastim within one year of transplant. Matching controlled for age, race, induction agent, and transplant type (deceased vs living donor). Primary endpoint was incidence of biopsy-proven acute rejection (BPAR) within 1 year of transplant.
Results: 42 out of 168 patients received filgrastim. Baseline demographics were similar, however there were more retransplants in the filgrastim group (9.5% vs 0%, p < 0.001). PRA was marginally higher in the filgrastim group (17.6% vs 9.6%, p=0.06). The incidence of 6-month BPAR was significantly higher in the filgrastim cohort, however the incidence of 12-month BPAR was not statistically significant. The total daily MMF dose was significantly lower at both 6 and 9 months in the filgrastim group. There was no difference in 12-month infection rate, however the incidence of CMV infection was higher in the filgrastim group.
| Filgrastim (N=42) | Control (N=126) | P-Value | |
| Any Infection 12 Mo,% | 31.7 | 36.4 | 0.6 |
| CMV viremia,% | 19.5 | 5.0 | 0.008 |
| BPAR 6 Mo,% | 19 | 5.6 | 0.008 |
| BPAR 12 Mo,% | 19 | 7.9 | 0.1 |
There was no difference in death-censored graft loss (HR 1.22, 95% CI 0.32-4.73).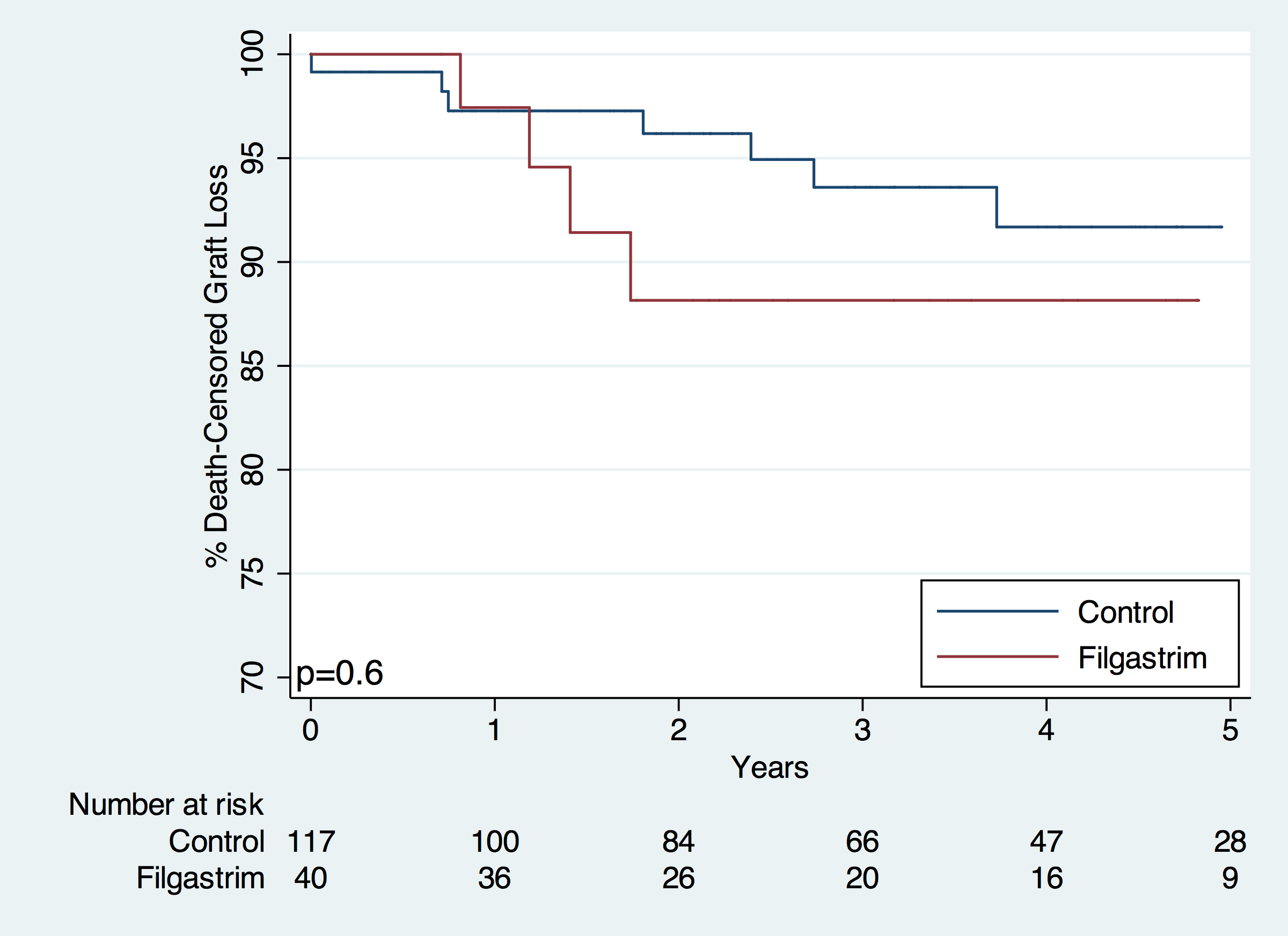 Conclusion: Renal transplant patients who receive filgrastim are at an increased risk for acute rejection within 6 months of transplant. Possible reasons for this include the immune stimulating effects of filgrastim and the lower doses of MMF seen in the filgrastim cohort. There were no significant differences in graft or patient survival.
Conclusion: Renal transplant patients who receive filgrastim are at an increased risk for acute rejection within 6 months of transplant. Possible reasons for this include the immune stimulating effects of filgrastim and the lower doses of MMF seen in the filgrastim cohort. There were no significant differences in graft or patient survival.
CITATION INFORMATION: Kane C, Lang K, Rendulic T, Goetz M, Evans R, Trobaugh K, Berger J. Impact of Filgrastim Use on Maintenance Doses of Immunosuppressants and Clinical Outcomes in Renal Transplant Patients. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Kane C, Lang K, Rendulic T, Goetz M, Evans R, Trobaugh K, Berger J. Impact of Filgrastim Use on Maintenance Doses of Immunosuppressants and Clinical Outcomes in Renal Transplant Patients. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-filgrastim-use-on-maintenance-doses-of-immunosuppressants-and-clinical-outcomes-in-renal-transplant-patients/. Accessed February 25, 2026.« Back to 2017 American Transplant Congress
