Chronic Hepatitis E-Genotype 3 Infection in Transplant Recipients: A Single Kidney and Pancreas Transplant Centre Experience.
1Renal and Transplant Centre, Imperial College NHS Trust, London, United Kingdom
2Department of Infection and Immunity, Imperial College NHS Trust, London, United Kingdom
3Department of Hepatology, Imperial College NHS Trust, London, United Kingdom
4Department of Hematology, Imperial College NHS Trust, London, United Kingdom
5Blood Borne Virus Unit, Virus Reference Department, MS-Colindale, Public Health England, London, United Kingdom
Meeting: 2017 American Transplant Congress
Abstract number: A182
Keywords: Blood transfusion, Infection, Liver
Session Information
Session Name: Poster Session A: Kidney Complications I
Session Type: Poster Session
Date: Saturday, April 29, 2017
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall D1
Introduction:
In the UK, Hepatitis E virus-genotype 3 (HEV3), is endemic and has now become the major cause of enterically transmitted viral hepatitis. Unlike HEV-genotype 1 found in developing countries, it can establish chronically in the immunosuppressed patient with potential complications such as cirrhosis and liver failure. The main mode of HEV3 transmission is through consumption of undercooked meat but parenteral transmission through blood products is well described.
Methods and Results:
Between 2010-2016, seven patients investigated for persistently raised ALTs were diagnosed with HEV infection. Retrospective analysis of stored blood samples identified 5 patients with chronic HEV3 infection (HEV-RNA detection for more than 3 months prior to diagnosis). Notably, 2 chronically infected-HEV patients had negative IgM and IgG testing, at the time of diagnosis. The source of infection was temporal with a transplant in 3 and dietary consumption in 4 patients. To date, 5 patients have been treated with ribavirin, 4 achieved HEV-clearance but 2 have relapsed.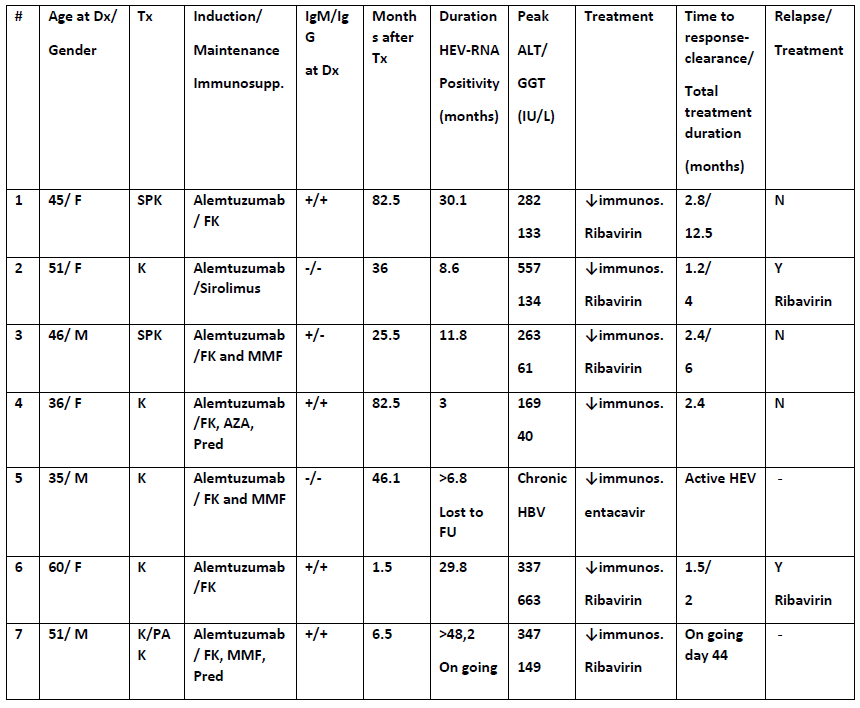 Discussion:
Discussion:
HEV3 infection should be suspected in all immunosuppressed patients with unexplained transaminitis and should be investigated by molecular testing as HEV serology is unreliable in this setting. The optimal treatment and duration remains to be determined. Blood products for transplant recipients should be HEV-tested. Further studies are needed to determine regional incidence and prevalence.
CITATION INFORMATION: Kousios A, Charif R, Sanchez E, Khan S, Regan F, Ijaz S, Muir D, Smith B, Tedder R, Taube D, Galliford J. Chronic Hepatitis E-Genotype 3 Infection in Transplant Recipients: A Single Kidney and Pancreas Transplant Centre Experience. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Kousios A, Charif R, Sanchez E, Khan S, Regan F, Ijaz S, Muir D, Smith B, Tedder R, Taube D, Galliford J. Chronic Hepatitis E-Genotype 3 Infection in Transplant Recipients: A Single Kidney and Pancreas Transplant Centre Experience. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/chronic-hepatitis-e-genotype-3-infection-in-transplant-recipients-a-single-kidney-and-pancreas-transplant-centre-experience/. Accessed January 7, 2026.« Back to 2017 American Transplant Congress
