Outcomes of 100 ABO Incompatible Kidney Transplants: A Single US Center Experience.
Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA
Meeting: 2017 American Transplant Congress
Abstract number: A55
Keywords: Highly-sensitized, HLA antibodies, Kidney transplantation, Outcome
Session Information
Session Name: Poster Session A: Clinical Science: Kidney Immunosuppression: Desensitization
Session Type: Poster Session
Date: Saturday, April 29, 2017
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall D1
Introduction
Acceptable patient and graft outcomes of ABO incompatible (ABOi) living donor kidney transplantation (LDKT) have been reported with the use of desensitization strategies. Data on impact of HLA sensitization on ABOi transplant outcomes is limited.
Methods
Patients who underwent ABOi LDKT at our center were included. Patients with T- or B-flow PRA≥30% were considered HLA sensitized. Patients underwent pre-transplant conditioning with MMF, rituximab x1, alternate day PLEX +IVIg 2 g/kg x1 with transplant within 1 week. Acceptable baseline and pre-transplant ABO antibody titers were ≤1:256 & ≤1:8 resp. Acceptable crossmatch (CXM) criteria for transplantation in ABOi/HLAi recipients included negative CDC CXM at 1:2 dilution, T and B-cell flow CXM ≤225MCS, and DSA ≤10,000MFI post-desensitization. Transplanted patients received induction with alemtuzumab and were maintained on tacrolimus/MMF/prednisone.
Results
From 1/2006 to 9/2016, 100 patients (Gp 1:82 (ABOi) vs Gp 2:18 (ABOi/HLAi)) underwent LDKT. Median follow up was 37.5 months. Flow CXM & DSA positivity at transplant was significantly higher in Gp 2. De novo DSA was more frequently detected in Gp 2. Acute rejection occurred as follows: ABMR 9.8% in Gp 1 vs 39% in Gp 2 (P=0.002); CMR 15% in Gp 1 vs. 28% in Gp 2 (P=NS).
| Gp 1 (ABOi) (n=82) | Gp 2 (ABOi/HLAi) (n=18) | P value | |
| Antibody titer at transplant, median (IQR) | 32 (8, 64) | 64 (32, 128) | 0.03 |
| DSA at transplant | 1 (1.2%) | 8 (44.4%) | <0.001 |
| Positive FCXM | 6 (7.3%) | 6 (33.3%) | 0.002 |
| Denovo DSA | 9 (11.0%) | 7 (38.9%) | 0.003 |
| ABMR | 8 (9.8%) | 7 (38.9%) | 0.002 |
| CMR | 12 (14.6%) | 12 (14.6%) | 0.18 |
| CABMR | 4 (4.9%) | 5 (27.8%) | 0.002 |
Patient, graft & antibody-mediated-rejection free survival were significantly lower in Gp 2. 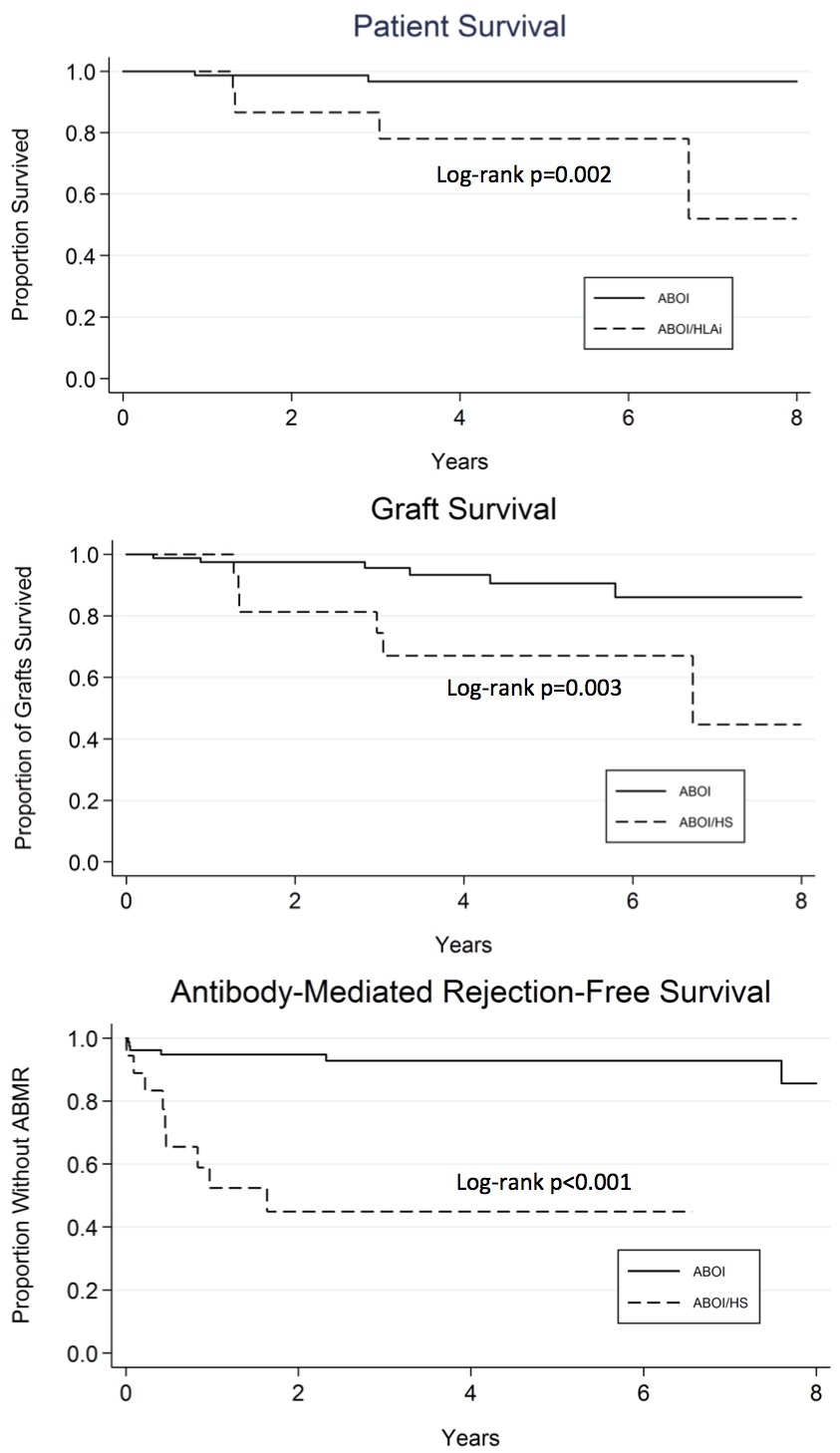 Conclusion
Conclusion
ABOi/HLAi patients are at high risk for adverse events after LDKT. Further research is needed to develop strategies for improving outcomes in these high immunological risk patients.
CITATION INFORMATION: Sethi S, Vo A, Huang E, Varanasi L, Choi J, Peng A, Najjar R, Jordan S. Outcomes of 100 ABO Incompatible Kidney Transplants: A Single US Center Experience. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Sethi S, Vo A, Huang E, Varanasi L, Choi J, Peng A, Najjar R, Jordan S. Outcomes of 100 ABO Incompatible Kidney Transplants: A Single US Center Experience. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/outcomes-of-100-abo-incompatible-kidney-transplants-a-single-us-center-experience/. Accessed February 26, 2026.« Back to 2017 American Transplant Congress
