Development of De Novo Donor Specific Antibodies (dnDSAs) Not the Presence of Pre-Transplant Donor Specific Antibodies (Pre-DSAs) Increase Rejection Risk in Crossmatch Negative Kidney Transplant Recipients.
Mayo Clinic Arizona, Phoenix, AZ
Meeting: 2017 American Transplant Congress
Abstract number: A19
Keywords: Antibodies, HLA antibodies, HLA matching, Rejection
Session Information
Session Name: Poster Session A: Antibody Mediated Rejection in Kidney Transplant Recipients I
Session Type: Poster Session
Date: Saturday, April 29, 2017
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall D1
Aim: Study the impact of Pre-DSAs on allograft outcomes in crossmatch negative kidney transplant recipients.
Methods: Single center retrospective analysis of characteristics and outcomes of kidney transplants with available HLA data 9/2011-4/2015. Single antigen bead studies were done pretransplant and at 4 months, 1 and 2 years. Flowcytometric crossmatch was done at transplant. For cause and surveillance biopsies were obtained at 4 months, 1 and 2 years and scored by Banff criteria. Antibodies were considered DSAs if MFIs>500. Follow up was through 8/2016. Antibodies posttransplant considered de novo if not present through pretransplant period.
Results: 774 recipients were included. 251 (32%) had pre-DSA, 52% Class I, 61% class II and 13% both. Median MFI for class I was 938, with 2.4% MFI>3000. For class II, median MFI was 1450 and 2.8% had MFI>3000. Pre-DSA group was not different from the rest by age, ESRD diagnosis, African American race, DGF, and graft loss or death. There were more females, retransplants, higher cPRA and more likely induced with rATG and maintained on prednisone. 23% of the Pre-DSA group developed dnDSA compared to 18% in the control p=.07. Rejection was higher in the Pre-DSA group Figure1. The development of dnDSA significantly impacted rejection risk Figure2.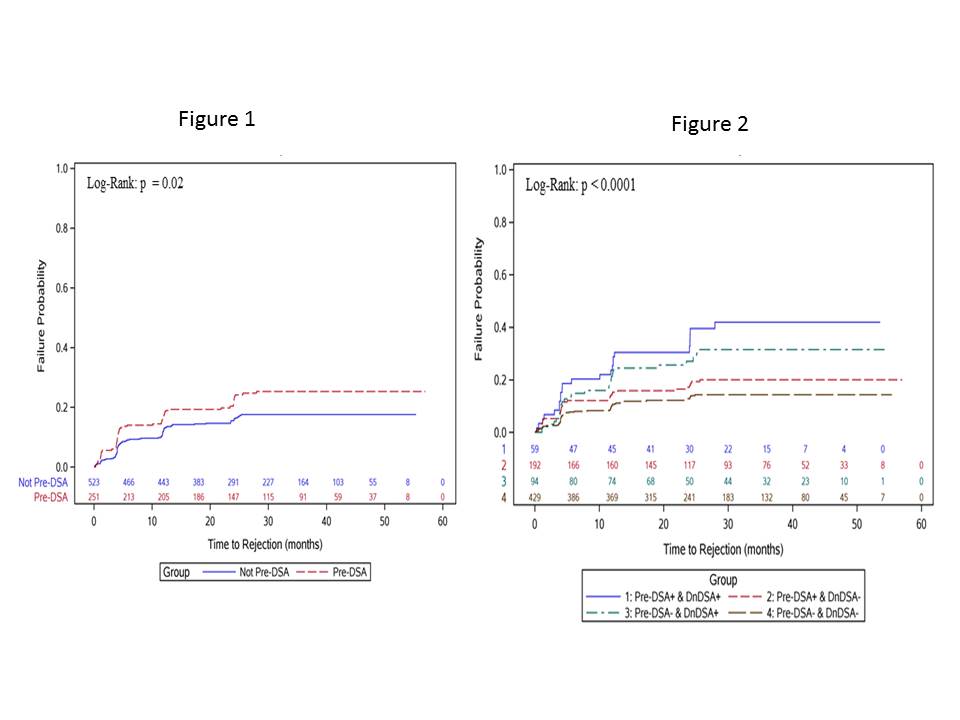 There was a significant increase in rejection hazard ratio in recipients who developed dnDSA regardless of their pre-DSA status.
There was a significant increase in rejection hazard ratio in recipients who developed dnDSA regardless of their pre-DSA status.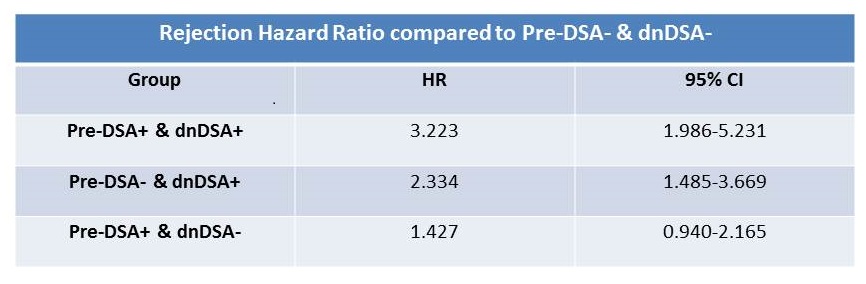 73% of all rejections were subclinical, 54% were Banff borderline cellular and only 10% were AMR.
73% of all rejections were subclinical, 54% were Banff borderline cellular and only 10% were AMR.
Conclusion: The presence of DSAs at time of transplant with negative crossmatch increased rejection rate but the risk was significantly influenced by the development of dnDSAs.
CITATION INFORMATION: Khamash H, Archambault L, Buras M, Kosiorek H, Pando M, Reddy K, Kaplan B, Heilman R. Development of De Novo Donor Specific Antibodies (dnDSAs) Not the Presence of Pre-Transplant Donor Specific Antibodies (Pre-DSAs) Increase Rejection Risk in Crossmatch Negative Kidney Transplant Recipients. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Khamash H, Archambault L, Buras M, Kosiorek H, Pando M, Reddy K, Kaplan B, Heilman R. Development of De Novo Donor Specific Antibodies (dnDSAs) Not the Presence of Pre-Transplant Donor Specific Antibodies (Pre-DSAs) Increase Rejection Risk in Crossmatch Negative Kidney Transplant Recipients. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/development-of-de-novo-donor-specific-antibodies-dndsas-not-the-presence-of-pre-transplant-donor-specific-antibodies-pre-dsas-increase-rejection-risk-in-crossmatch-negative-kidney-transplant-recip/. Accessed January 15, 2026.« Back to 2017 American Transplant Congress
