Impact of Antihypertensive (AHT) Choice and Advagraf® Dose on Immunologic Events in Renal Allografts 24 Months Post-Transplantation (Tx).
1Health Sciences Centre, Winnipeg, MB, Canada
2University of Alberta Hospital, Edmonton, AB, Canada
3Astellas Pharma Global Development, Northbrook, IL
4Astellas Pharma Global Development, Markham, ON, Canada
Meeting: 2017 American Transplant Congress
Abstract number: 71
Keywords: Antibodies, Inflammation, Kidney transplantation, Rejection
Session Information
Session Name: Concurrent Session: Novel Immunosuppression Regimens - Tacrolimus Combinations
Session Type: Concurrent Session
Date: Sunday, April 30, 2017
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:06pm-3:18pm
Presentation Time: 3:06pm-3:18pm
Location: E450b
Renal allograft inflammation may be modulated by ACEi- or ARB-type AHTs (renin-angiotensin system (RAS)-targeting drugs that may inhibit calcineurin) or by optimizing tacrolimus (TAC) exposure. The effect on immunologic events of combined RAS blockade and reduced-dose Advagraf® (prolonged-release TAC) is unknown.
Multicenter prospective open-label controlled trial in adult de novo renal Tx patients (pts) randomized in 2×2 design to ACEi/ARBs vs other (O) AHTs (Mo 1-24) and to low TAC (LOW; target trough 5±1ng/mL to Mo 6) vs standard TAC (STD; target trough reduced from 12±2 to 8±2ng/mL by Mo 6). All pts received basiliximab, MMF and steroids. TAC target after Mo 6 was by physician discretion. T-cell mediated rejection with borderline change (TCMR/B), TCMR and donor-specific antibody (DSA) development were prespecified secondary outcomes. Follow-up of secondary outcomes is planned at Yr 5 post-Tx.
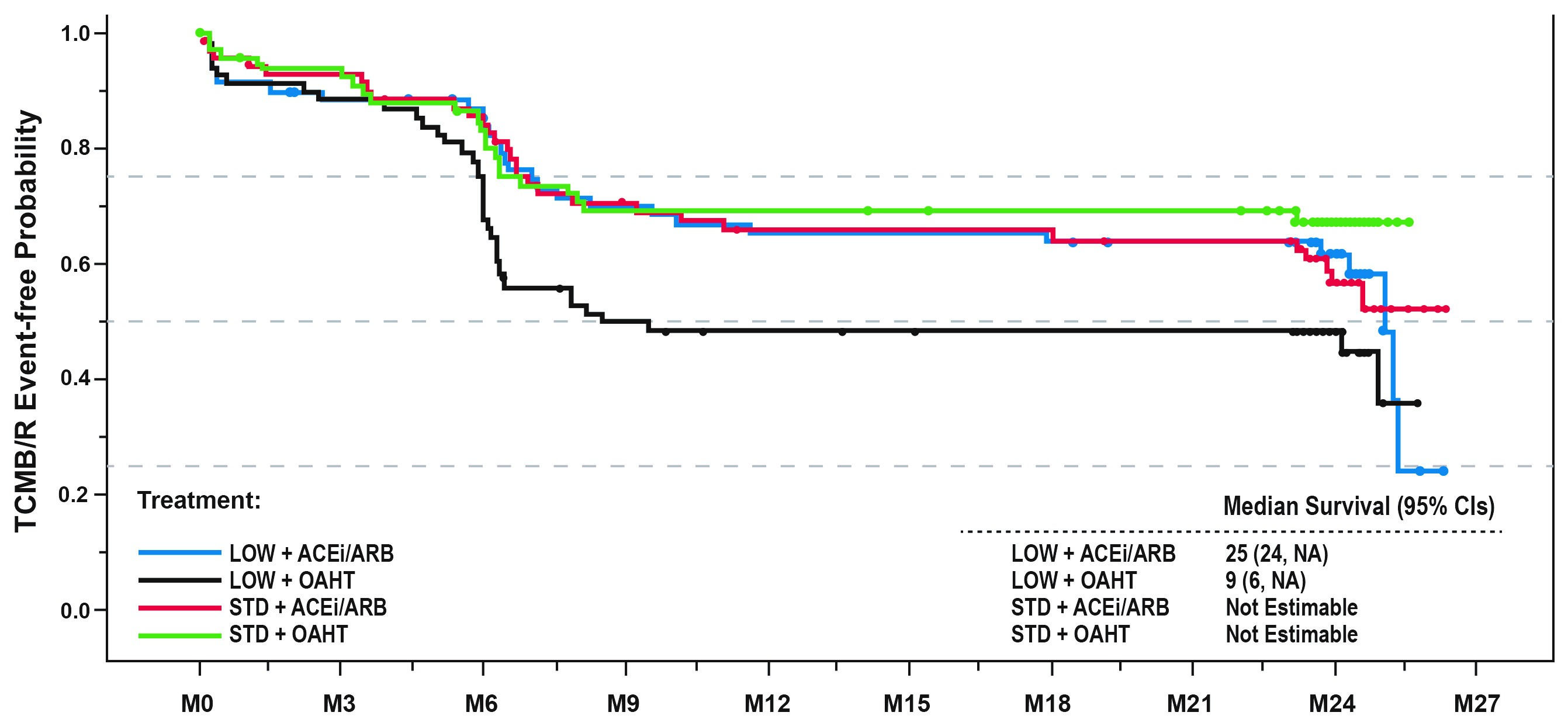
Of 281 pts in the ITT population, 235 remained in the study by Mo 24. Time to 1st TCMR/B did not differ significantly between ACEi/ARB and OAHT pts but was delayed for STD vs LOW TAC pts (HR [95% CI]: 2.115 [1.002, 4.462], P=0.0493). Among LOW TAC-treated pts, time to 1st TCMR/B was delayed for ACEi/ARB vs OAHT (Figure 1). Similar trends were evident for time to TCMR. Donor age >50 yrs was also associated with TCMR/B, relative to pts ≤50 (HR [95% CI]: 1.862 [1.267, 2.740], P=0.0016). De novo DSAs were found at Mo 24 in 5.2% for ACEi/ARB vs 6.0% for OAHT pts and 3.8% for STD vs 7.4% for LOW TAC pts. Blood pressure, eGFR and urinary protein loss were similar over 24 Mo for all treatment groups.
LOW TAC was associated with higher TCMR/B risk by Mo 6 and numerically greater DSA incidence by Mo 24, relative to STD TAC. AHT choice did not affect TCMR/B risk with STD TAC, but RAS-blocking AHTs may reduce cellular rejection risk with LOW TAC. Long-term implications of these results will be evaluated at Yr 5.
Figure 1: Time to first TCMR/B
CITATION INFORMATION: Rush D, Cockfield S, Wilson S, Howell J, FKC-014 Study Group Impact of Antihypertensive (AHT) Choice and Advagraf® Dose on Immunologic Events in Renal Allografts 24 Months Post-Transplantation (Tx). Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Rush D, Cockfield S, Wilson S, Howell J, Group FKC-014Study. Impact of Antihypertensive (AHT) Choice and Advagraf® Dose on Immunologic Events in Renal Allografts 24 Months Post-Transplantation (Tx). [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-antihypertensive-aht-choice-and-advagraf-dose-on-immunologic-events-in-renal-allografts-24-months-post-transplantation-tx/. Accessed February 3, 2026.« Back to 2017 American Transplant Congress