Initial Experience Treating Hepatitis C Positive Kidney Transplant Recipients with Non-Interferon-Containing Regimens: Successes and Challenges.
Weill Cornell Medical College, New York, NY.
Meeting: 2016 American Transplant Congress
Abstract number: 72
Keywords: Hepatitis C, Kidney transplantation, Liver
Session Information
Session Name: Concurrent Session: SOT: HIV, HBV, & HCV
Session Type: Concurrent Session
Date: Sunday, June 12, 2016
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:30pm-2:42pm
Presentation Time: 2:30pm-2:42pm
Location: Room 313
Historically, treatment of hepatitis C virus (HCV) infection with interferon-based regimens after kidney transplantation has been associated with risk of rejection & graft loss. Direct acting antiviral (DAA) regimens now provide opportunity to cure patients of HCV, potentially leading to improved post-transplant outcomes.
Methods: We describe our transplant center's initial experience utilizing DAA regimens to treat HCV(+) patients after kidney transplantation.
Results: At the time of submission, 29 deceased donor kidney transplant recipients have begun &/or completed anti-HCV therapy with a DAA regimen at a median of 29 months post-transplant (range 2-122). 89% are male with median age of 59 at transplant (range 37-83); 85% are HCV genotype 1, median HCV viral load (VL) at transplant was 1.5 million (M) (range 77K-68M), 70% were HCV treatment naive, & 70% received a HCV(+) kidney, which significantly reduced waiting time (595 vs. 1461 days (p=0.01)). Over 80% received a Thymo induction/steroid-sparing regimen at transplant; median HCV VL at therapy start was 4.5M (range 364K-37M). eGFR & fibrosis at therapy start are seen in Figure 1a & b, while DAA regimens utilized are seen in Figure 1c.
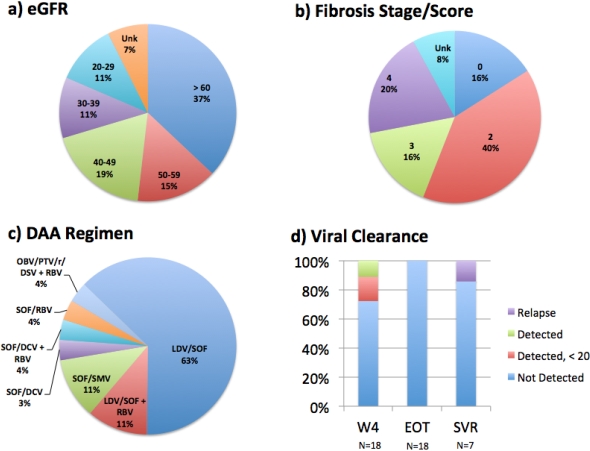
To date, 13/18 (72%) cleared HCV by week 4 (Figure 1d), 18/18 (100%) cleared HCV by end of therapy (EOT) & 6/7 (86%) achieved sustained viral response (SVR) at 12 weeks following EOT; 5/6 with SVR had received a HCV(+) donor kidney. 1 patient with HCC relapsed after being negative at EOT.
Two patients self-discontinued DAA's due to side effects & HCC diagnosis. Adverse events included anemia requiring RBV dose reduction or discontinuation (n=2), headache (n=2), acute kidney injury due to tacrolimus toxicity, diarrhea, & worsening blood glucose control (n=1 each). One patient died 4 months after achieving SVR of an unknown cause.
Conclusion: Treatment of HCV after kidney transplantation is now a reality. Cautious selection of regimen based on renal function, interacting medications, & adverse event profile is warranted. Long-term follow-up is needed to assess the impact on transplant outcomes.
CITATION INFORMATION: Aull M, Watkins A, Kim J, Rhee K, Dadhania D, Lee J, Kapur S. Initial Experience Treating Hepatitis C Positive Kidney Transplant Recipients with Non-Interferon-Containing Regimens: Successes and Challenges. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Aull M, Watkins A, Kim J, Rhee K, Dadhania D, Lee J, Kapur S. Initial Experience Treating Hepatitis C Positive Kidney Transplant Recipients with Non-Interferon-Containing Regimens: Successes and Challenges. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/initial-experience-treating-hepatitis-c-positive-kidney-transplant-recipients-with-non-interferon-containing-regimens-successes-and-challenges/. Accessed February 21, 2026.« Back to 2016 American Transplant Congress
