A Model to Explore the Potential Budget Impact of a Novel Screening Tool for the Detection of Subclinical Rejection Among Kidney Transplant Patients.
1Immucor, Inc, Waukesha, WI
2Avalare Health, LLC, Washington, DC.
Meeting: 2016 American Transplant Congress
Abstract number: D188
Keywords: Economics, Graft survival, Monitoring, Rejection
Session Information
Session Name: Poster Session D: Organizational and Operational Aspects of Transplantation
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Background: Acute rejection among kidney transplant patients is common, with ~10% of individuals experiencing acute rejection within the first year and rising to 16-17% at five years. Routine monitoring includes serum creatinine levels, but this is considered to be nonspecific for acute rejection and is only detected after substantial damage has occurred. The Kidney Solid Organ Response Test (kSORT) assay is a non-invasive molecular assay that measures blood-based gene markers for transplanted kidney rejection. In the Acute Rejection in Renal Transplantation (AART) study, acute rejection was detected by kSORT up to 3 months before detection by biopsy, giving providers an earlier opportunity to modify immunosuppression to prevent subsequent rejection. The objective of this analysis is to evaluate the potential budget impact of the kSORT assay for rejection surveillance from a commercial payer perspective. Methods: A 2-year exploratory semi-Markov cohort model with monthly cycles was constructed to model the relationship between subclinical rejection (SCR), acute cellular rejection (ACR), and graft failure (GF). Four separate Markov models represent no monitoring, monitoring with protocol biopsy(PB) only, monitoring with kSORT only, and monitoring with PB and kSORT, while incorporating the months during which a patient receives the respective monitoring tool (i.e., kSORT, PB, or both). Results: In the base case scenario, kSORT is expected to produce a budget impact of $0.0057 per member per month (PMPM) during the first year and $0.0058 PMPM during the second year. 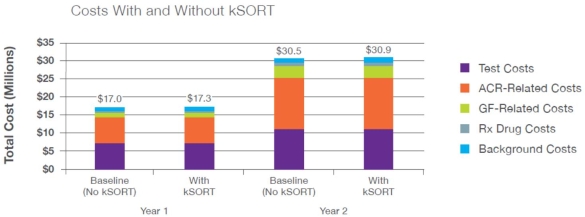 Conclusions: This model indicates that the kSORT assay is expected to produce minimal budget impact to payers. The low budget impact is attributed to the small patient population within plans with renal transplants every year, in addition to relatively low acute rejection and graft failure rates. Although the budget impact is small, additional clinical data showing how kSORT improves patient outcomes are needed. Long-term data may be of particular import in showing true value of the technology.
Conclusions: This model indicates that the kSORT assay is expected to produce minimal budget impact to payers. The low budget impact is attributed to the small patient population within plans with renal transplants every year, in addition to relatively low acute rejection and graft failure rates. Although the budget impact is small, additional clinical data showing how kSORT improves patient outcomes are needed. Long-term data may be of particular import in showing true value of the technology.
CITATION INFORMATION: Jaglinski K, Uchida H, Inocencio T, Hughes K. A Model to Explore the Potential Budget Impact of a Novel Screening Tool for the Detection of Subclinical Rejection Among Kidney Transplant Patients. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Jaglinski K, Uchida H, Inocencio T, Hughes K. A Model to Explore the Potential Budget Impact of a Novel Screening Tool for the Detection of Subclinical Rejection Among Kidney Transplant Patients. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/a-model-to-explore-the-potential-budget-impact-of-a-novel-screening-tool-for-the-detection-of-subclinical-rejection-among-kidney-transplant-patients/. Accessed January 8, 2026.« Back to 2016 American Transplant Congress
