Antithymocyte (ATG) Dose Density in Renal Transplant Recipients (RTR) with Early Graft Dysfunction (EGD).
Houston Methodist Hospital, Houston, TX.
Meeting: 2016 American Transplant Congress
Abstract number: C51
Keywords: Induction therapy, Kidney transplantation, Rejection
Session Information
Session Name: Poster Session C: Clinical Science - Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Monday, June 13, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
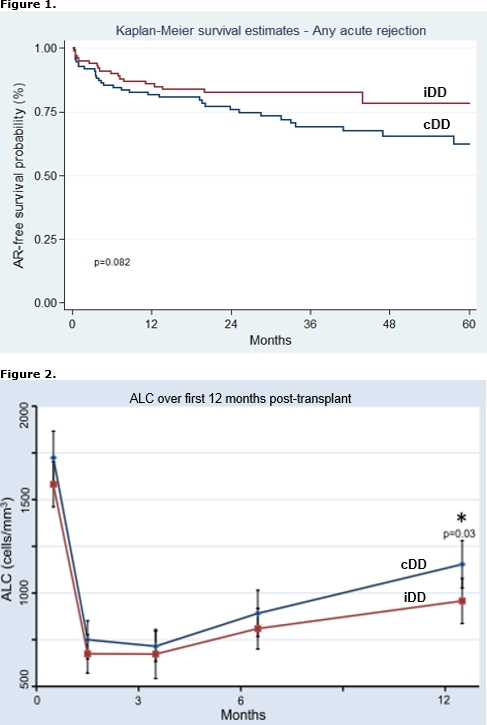
The cumulative amount of ATG for induction may be given as consecutive (cDD) or intermittent day dosages (iDD). iDD allows for leukocyte or platelet recovery or delay in calcineurin inhibitor (CNI) initiation for RTR with EGD. The purpose of this study was to compare graft outcomes in RTR with EGD who received cDD to those with iDD. EGD was defined as a <30% reduction in SCr from post-operative day (POD) 1 to 2 or need for dialysis (DGF). A total of 210 RTR from 1/2008 to 8/2014 with EGD were reviewed, including 60 with DGF. Baseline demographics were similar; however, iDD patients had higher rates of DGF (47% vs. 12%), received more total ATG over a longer period (5.8±1.2 vs. 4.9±0.8 mg/kg and 5.8±1.7 vs. 3.3±0.5 days, respectively), started CNI later (6.7±2.4 vs. 4.5±2.6 days), and had a longer length of stay (8.9±3.8 vs. 6.2±3.8 days) than RTR with cDD [p<0.01 for all comparisons]. iDD patients experienced lower rates of AR [Figure 1]. This was predominately due to less AR in patients without DGF (15% vs. 30%, p=0.03). By multivariate analysis, iDD was associated with a lower risk for AR, independent of cumulative ATG dose and other risk factors for AR [Table 1]. Absolute lymphocyte count (ALC) was similar at 1, 3, and 6 months but lower in iDD patients at 12 months (957±570 vs. 1153±640, p=0.03) [Figure 2]. GFR at 1, 3, 6, and 12 months was similar between groups. Our data suggests that iDD may reduce AR in EGD, possibly due to more prolonged lymphocyte depletion. Formal investigation into ATG dose density in the setting of EGD is warranted.
| Table 1. Risk Factors for AR | Univariate analysis | Multivariate analysis | |||
| No AR (n=158) | AR (n=52) | p-value | HR (95% CI) | p-value | |
|
Age, years |
50±12 | 48±15 | 0.03 | 0.98 (0.96-1) | 0.04 |
| African American (AA), n(%) | 75 (48%) | 33 (64%) | 0.06 | 1.86 (1.05-3.31) | 0.03 |
| Deceased donor, n(%) | 137 (87%) | 47 (90%) | 0.46 | ||
| PRA, % | 34±34 | 44±35 | 0.10 | 1.01 (1-1.02) | 0.03 |
| DGF, n(%) | 45 (29%) | 15 (29%) | 0.96 | ||
| Cumulative ATG dose, mg/kg | 5.3±1.2 | 5.2±4.9 | 0.51 | ||
| iDD, n(%) | 82 (52%) | 19 (37%) | 0.08 | 0.5 (0.27-0.94) | 0.03 |
CITATION INFORMATION: Kuten S, Patel S, Knight R, Nguyen D, Graviss E, Podder H, Gaber A. Antithymocyte (ATG) Dose Density in Renal Transplant Recipients (RTR) with Early Graft Dysfunction (EGD). Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Kuten S, Patel S, Knight R, Nguyen D, Graviss E, Podder H, Gaber A. Antithymocyte (ATG) Dose Density in Renal Transplant Recipients (RTR) with Early Graft Dysfunction (EGD). [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/antithymocyte-atg-dose-density-in-renal-transplant-recipients-rtr-with-early-graft-dysfunction-egd/. Accessed February 23, 2026.« Back to 2016 American Transplant Congress
