Pharmakokinetics of Prolonged-Release Formulation of Tacrolimus: Comparison of AUC0-24 and Concentration at 10 Time Points.
1Nephrology, Tokyo Women's Medical University, Tokyo, Japan
2Urology, Tokyo Women's Medical University, Tokyo, Japan.
Meeting: 2016 American Transplant Congress
Abstract number: C39
Keywords: Calcineurin, Immunosuppression, Kidney transplantation, Pharmacokinetics
Session Information
Session Name: Poster Session C: Clinical Science - Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Monday, June 13, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Background:Use of an appropriate immunosuppressant is necessary for good graft survival in kidney transplantation (KTx). Currently, a prolonged-release formulation of tacrolimus (Graceptor or Adovagraf, Astellas Pharma; TAC-ER) has been recently developed and widely used. In the clinical setting, trough concentration of TAC-ER (C0) was generally evaluated and the dosage of TAC-ER was adjusted. We previously reported the correlation between twenty-four hour area under the curve (AUC0-24) and C0, and found that C0 was well correlated with AUC0-24. However, the sample size studied was small (50 patients). Further, no other studies reported this correlation; therefore, the actual pharmacokinetics (PK) of TAC-ER is yet to be determined. Here, we evaluated the correlation between AUC0-24 and concentration of TAC-ER.
Methods: During the period between January 2011 and August 2015, 309 KTx recipients were enrolled in PK study and underwent renal biopsy on post-operative day 14 at our institution. Blood samples were collected at 10 time points (C0, C1-4, C12-16).
Results: AUC0-24≥250 ng[bull]h/mL was observed in 222 patients and AUC0-24≺250 was observed in 87 patients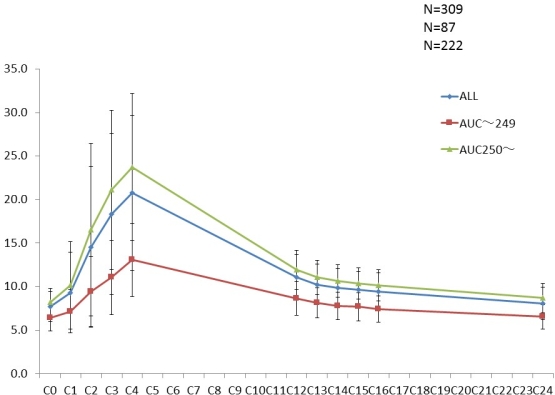 . There was a significant correlation between AUC0-4 and AUC0-24 (R2=0.6413). C4 was well correlated with AUC0-24 (R2=0.6759)
. There was a significant correlation between AUC0-4 and AUC0-24 (R2=0.6413). C4 was well correlated with AUC0-24 (R2=0.6759)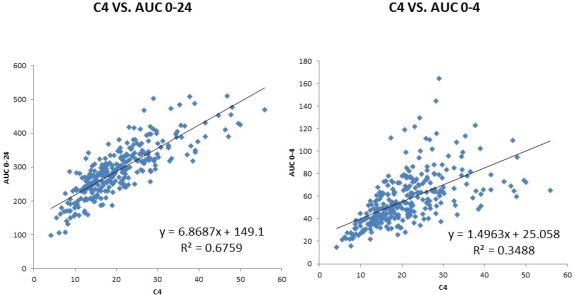 ; however, C0 was less correlated with AUC0-24 (R2=0.2973). Renal biopsy showed that CNI toxicity was marked in the AUC0-24≥250 group compared to that in the ACU0-24<250 group.
; however, C0 was less correlated with AUC0-24 (R2=0.2973). Renal biopsy showed that CNI toxicity was marked in the AUC0-24≥250 group compared to that in the ACU0-24<250 group.
Conclusions: C4 had a significant correlation with AUC0-24, indicating good concentration monitoring. This is the first report of the pharmacokinetics of TAC-ER in a large population.
CITATION INFORMATION: Unagami K, Okumi M, Furusawa M, Hirai T, Shimizu T, Omoto K, Inui M, Ishida H, Nitta K, Tanabe K. Pharmakokinetics of Prolonged-Release Formulation of Tacrolimus: Comparison of AUC0-24 and Concentration at 10 Time Points. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Unagami K, Okumi M, Furusawa M, Hirai T, Shimizu T, Omoto K, Inui M, Ishida H, Nitta K, Tanabe K. Pharmakokinetics of Prolonged-Release Formulation of Tacrolimus: Comparison of AUC0-24 and Concentration at 10 Time Points. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/pharmakokinetics-of-prolonged-release-formulation-of-tacrolimus-comparison-of-auc0-24-and-concentration-at-10-time-points/. Accessed February 15, 2026.« Back to 2016 American Transplant Congress
