Randomized Controlled Trial of Mixed Acute Rejection Therapy in Renal Allografts: 3 Year Follow Up.
1U. of Cincinnati, Cincinnati
2The Christ Hospital, Cincinnati.
Meeting: 2016 American Transplant Congress
Abstract number: C2
Keywords: Rejection
Session Information
Session Name: Poster Session C: Antibody Mediated Rejection: Session #1
Session Type: Poster Session
Date: Monday, June 13, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Optimal therapy for mixed acute rejection (MAR) (defined as coexisting acute cellular and antibody mediated rejection) has not been defined. Rabbit antithymocyte globulin (rATG) has frequently been used to treat MAR as histopathology is often dominated by acute cellular rejection features. The purpose of this study was to evaluate the addition of rituximab (RTX) or bortezomib (BTZ) to rATG for MAR treatment.
Kidney transplant patients (pts) were randomized to receive rATG, rATG+RTX (375mg/m2), or rATG+BTZ (1.3mg/m2 x 4). rATG was dosed to suppress CD3 for 7-14 days based on Banff ACR grade. All pts received 3 pheresis sessions after treatment. For pts with <50% reduction in immunodominant donor specific antibody (iDSA) at 7, 30, 60 or 90 days, BTZ consolidation therapy (1.3mg/m2 x 4) was considered. The primary endpoints were rejection reversal at 14 days and recurrent rejection within 90 days. Rejection reversal was defined as a return of serum creatinine (Scr) to 115% of baseline or histologic reversal.
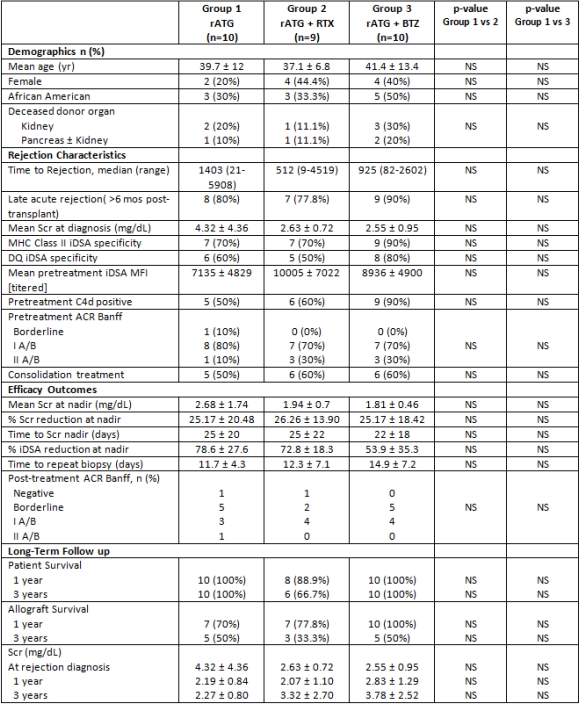
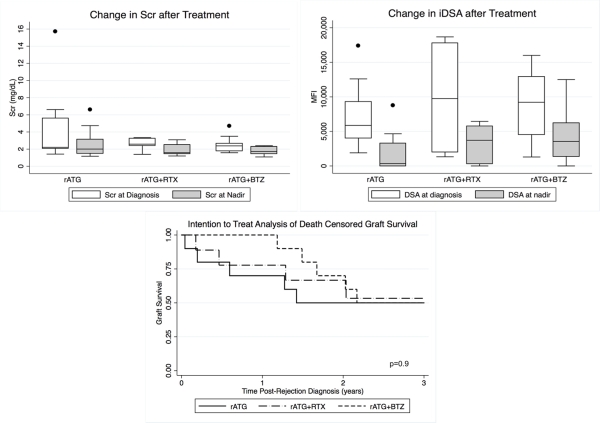
30 MAR episodes in 29 pts were treated. Most MAR episodes were late (>6 months post-transplant) and associated with a de novo DQ donor specific antibody. 4 pts had rejection reversal at 14 days by renal function criteria. 13 additional pts had histologic improvement on repeat biopsy. Recurrent rejection occurred in 2 pts; both pts received BTZ. The most common adverse effect was anemia; hemoglobin <8 g/dL occurred in 13 pts. 7 of these pts received BTZ consolidation therapy. Renal function, graft survival, and DSA reduction were similar between groups.
No statistically significant differences in graft survival were observed at 3 years between treatment groups. Larger experiences are necessary to determine optimal MAR treatment.
 .
.
 .
.
CITATION INFORMATION: Leino A, Lichvar A, Abu-Jawdeh B, Govil A, Mogilishetty G, Cardi M, Kremer J, Cuffy M, Paterno F, Alloway R, Shields A, Woodle E. Randomized Controlled Trial of Mixed Acute Rejection Therapy in Renal Allografts: 3 Year Follow Up. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Leino A, Lichvar A, Abu-Jawdeh B, Govil A, Mogilishetty G, Cardi M, Kremer J, Cuffy M, Paterno F, Alloway R, Shields A, Woodle E. Randomized Controlled Trial of Mixed Acute Rejection Therapy in Renal Allografts: 3 Year Follow Up. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/randomized-controlled-trial-of-mixed-acute-rejection-therapy-in-renal-allografts-3-year-follow-up/. Accessed February 11, 2026.« Back to 2016 American Transplant Congress
