Infusion of Polyclonal Tregs Modulates Subclinical Kidney Transplant Inflammation: Final Report of TASK Pilot Trial.
UCSF, San Francisco
Meeting: 2017 American Transplant Congress
Abstract number: 68
Keywords: Inflammation, Kidney transplantation, Protocol biopsy
Session Information
Session Name: Concurrent Session: Novel Immunosuppression Regimens - Tacrolimus Combinations
Session Type: Concurrent Session
Date: Sunday, April 30, 2017
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:30pm-2:42pm
Presentation Time: 2:30pm-2:42pm
Location: E450b
Purpose:
Treg therapy can reverse established inflammation in animal models. We conducted a pilot safety & feasibility trial of Treg therapy for subclinical kidney transplant inflammation.
Methods:
Peripheral blood CD4+CD25+CD127lo/- Tregs were isolated (FACS) & polyclonally expanded ex vivo in medium containing deuterated glucose. 320×106 Tregs were infused into kidney transplant recipients with subclinical inflammation on 6-m protocol biopsy. Levels of infused (labeled) Tregs in circulation were tracked by measuring deuterium using GC-MS. Inflammation was assessed on follow-up biopsies using Banff scoring and leukocyte common antigen positive (LCA+) cell density. Urine was assayed for common rejection module (uCRM), a biomarker of rejection.
Results:
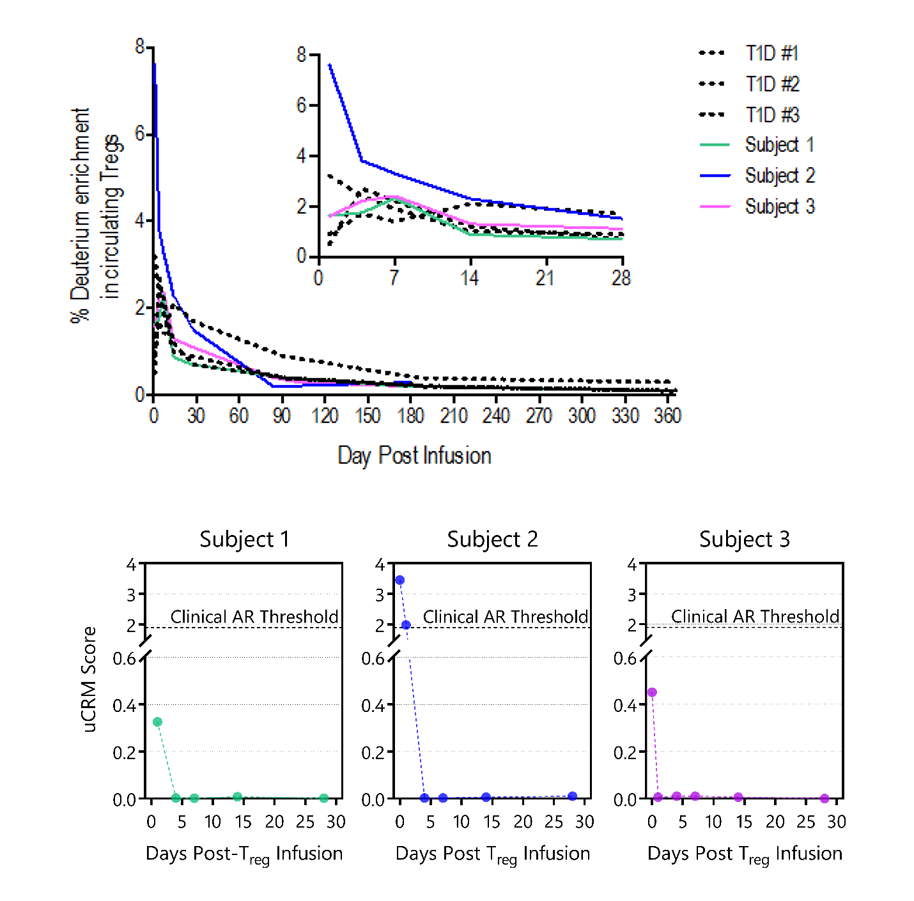
3 transplant recipients on tac/MMF/prednisone received Treg infusions and have completed 1 year of follow up. Tregs expanded >100-fold & met all release criteria for infusion. One patient had transient lymphopenia (day 4-21) post-infusion. No infusion reactions or infections were seen & graft function remained stable. Infused Tregs peaked within week 1, making up to 7.5% of all circulating Tregs and their pharmacokinetics was similar to that seen in non-immunosuppressed patients (figure). Graft inflammation & uCRM scores improved (table and figure).
Conclusions:
It is feasible to isolate & expand Tregs from transplanted patients. Infused Tregs were well tolerated & had pharmacokinetics similar to those seen in non-immunosuppressed patients. Modulation of graft inflammation was seen post-infusion. CTOT-21 will further test the efficacy of Tregs for control of graft inflammation.
| Subject | Banff scores | LCA+ cells/mm2 | ||
| Baseline | 2 weeks | Baseline | 2 weeks | |
| 1 | i1t1 | i0t0 | 337.2 | 18.9 |
| 2 | i0t1 | i0t0 | 469.1 | 303.5 |
| 3 | i1t1 | i0t1 | 278.0 | 454.4 |
CITATION INFORMATION: Chandran S, Tang Q, Sarwal M, Laszik Z, Putnam A, Sigdel T, Tavares E, Bluestone J, Vincenti F. Infusion of Polyclonal Tregs Modulates Subclinical Kidney Transplant Inflammation: Final Report of TASK Pilot Trial. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Chandran S, Tang Q, Sarwal M, Laszik Z, Putnam A, Sigdel T, Tavares E, Bluestone J, Vincenti F. Infusion of Polyclonal Tregs Modulates Subclinical Kidney Transplant Inflammation: Final Report of TASK Pilot Trial. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/infusion-of-polyclonal-tregs-modulates-subclinical-kidney-transplant-inflammation-final-report-of-task-pilot-trial/. Accessed January 18, 2026.« Back to 2017 American Transplant Congress