Variation in Next of Kin Authorization for Research in Deceased Donor Organ Transplantation: Experience at One Organ Procurement Organization
1Saint Louis University, St. Louis, MO, 2Mayo Clinic, Rochester, MN, 3Mid-America Transplant, St. Louis, MO, 4University of Miami, Miami, FL, 5University of Nebraska, Omaha, NE
Meeting: 2021 American Transplant Congress
Abstract number: 1253
Keywords: African-American, Donation, Ethics, Hispanic
Topic: Clinical Science » Organ Inclusive » Deceased Donor Management and Intervention Research
Session Information
Session Name: Deceased Donor Management and Intervention Research
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Research related to deceased donor interventions seeks to address diverse questions to improve the use and outcomes of organ gifts for transplantation. Consent for deceased donor research is obtained from the next of kin (NOK) authorizing donation or through first-person authorization (FPA), but to date, there is limited information on patterns of research authorization or how decline rates may impact access to new information for certain groups.
*Methods: We performed a retrospective chart review of all deceased organ donors from 1/2017-12/2019 at a large Midwestern organ procurement organization. We determined FPA authorization status, NOK relationship, and final research authorization using the Critical Organ documents and FPA registration records. Multivariable logistic regression analysis was used to assess the association between donor factors and research authorization (adjusted odds ratio, 95% LCL aOR 95% UCL).
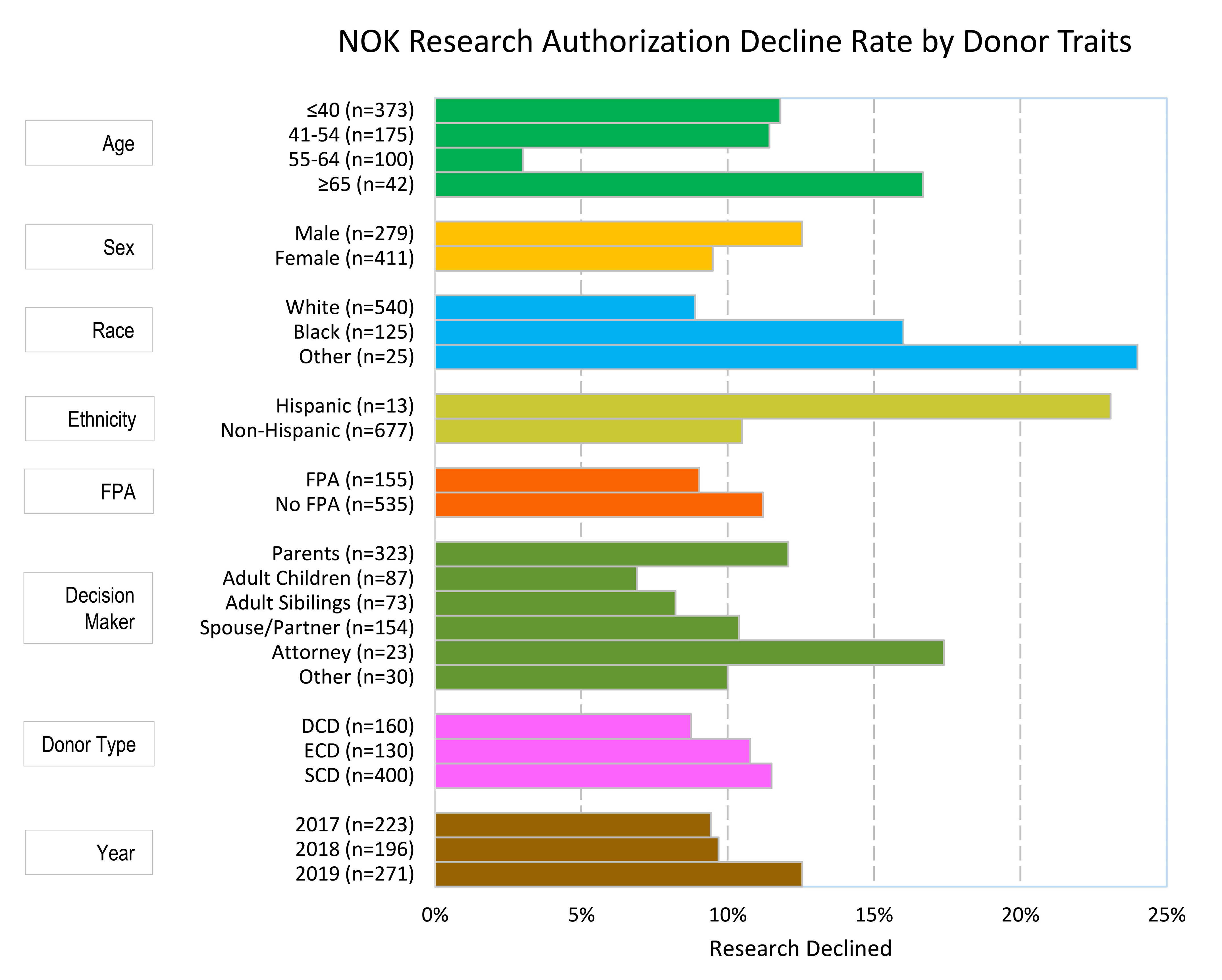
*Results: Medical records for 688 deceased donors in the study period were reviewed. Of these, 659 (95.5%) had an organ recovered for transplant. Overall, research authorization was provided in 89.2% of donations and declined in 10.8%. Compared to white donors, research decline was higher for Black (16.0% vs 8.9%; aOR, 1.051.903.43, p=0.05) and other non-white donors (24.0% vs 8.9%; aOR, 1.213.319.06, p=0.05). Unadjusted research decline was higher for Hispanic donors (23.1% vs 10.5%), but decline was not significantly different with adjustment. Compared to donors age <40, research decline was lower for donors aged 55-64 (3.0% vs 11.8%; aOR, 0.07 0.240.84, p=0.05), but higher for donors age 65 and older (16.7% vs 11.8%; aOR, 0.953.6113.79, p=0.05). Ultimate research decline trended lower when donor had provided FPA (9.0% vs 11.2%; aOR, 0.240.511.08, p=0.05). There was no significant association of research authorization with NOK relationship to donor, donor sex, donor type, or year of donation.
*Conclusions: Deceased donor research authorization decline is higher for Black, other non-white donors, and donors older than age 65. These findings may inform educational interventions for both staff and families to improve research authorization rates and increase opportunities to provide science for vulnerable groups.
To cite this abstract in AMA style:
Lentine KL, Jones C, Cheungpasitporn W, Rothweiler R, Xiao H, Ortigosa-Goggins M, Marklin G, Mannon RB. Variation in Next of Kin Authorization for Research in Deceased Donor Organ Transplantation: Experience at One Organ Procurement Organization [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/variation-in-next-of-kin-authorization-for-research-in-deceased-donor-organ-transplantation-experience-at-one-organ-procurement-organization/. Accessed February 16, 2026.« Back to 2021 American Transplant Congress