Validation of Urine CXCL10 for Diagnosis of Subclinical and Clinical Rejection in Pediatric Kidney Transplants
University of Manitoba, Winnipeg, Canada
Meeting: 2013 American Transplant Congress
Abstract number: A657
Non-invasive testing for detection of acute rejection is needed to improve outcomes. Urinary CXCL10 has been validated as a biomarker of tubulitis in adults and we hypothesize that it is a robust marker in children.
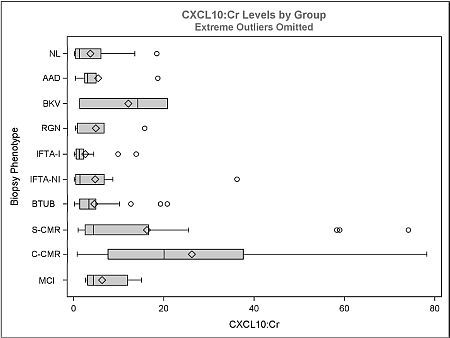
Urine samples (n=140) from 51 patients with concurrent surveillance or indication biopsies were classified according to histology and assayed for urinary CXCL10 using ELISA. The groups analyzed were: Normal (NL=21), acute allograft dysfunction (NL histology, ↑Cr >25%; AAD=6), BKV nephritis (BKV=3), recurrent glomerulonephritis (RGN=5), interstitial fibrosis/tubular atrophy with/without inflammation (ITFA-I=15, IFTA-NI=16), borderline tubulitis (BTUB=36), subclinical cell-mediated rejection (SCMR=17), clinical CMR (CCMR=9), and microcirculatory inflammation (≥g1ptc1, i0t0; MCI=12). Individual Banff scores were correlated with CXCL10. Subclinical cohort (n=104) defined by <25% ↑Cr from baseline.
The ratio of CXCL10:Cr distinguishes CCMR from NL, AAD, IFTA-I, IFTA-NI, RGN and BTUB (Figure, p<0.05); and SCMR from NL, IFTA-I, IFTA-NI, RGN and BTUB (p<0.05). CCMR did not differ significantly from SCMR, MCI and BKV. BTUB did not differ from NL, IFTA-I, IFTA-NI. CXCL10:Cr correlated with Banff i, t, g and ptc scores only and not allograft function (p<0.05). Excluding BTUB, MCI, BKV and RGN, the area under ROC curve to distinguish 'other' vs. all CMR (SCMR+CCMR) was 0.81 (p=0.02) and subclinical 'other' vs. SCMR was 0.81 (p=0.04). Sensitivity/specificity was 77/60% and 59/67% with a CXCL10:Cr cut-off of 4.7 and 4.8 ng/mmol respectively. The 4.7 ng/mmol cutoff also identifies MCI (67%), BKV (67%), RGN (40%) and BTUB (33%). 80% sensitivity is achieved for all CMR and SCMR with cutoffs of 3.1 and 3.0 ng/mmol respectively. 80% specificity is achieved with cutoffs of 10.1 and 8.1 ng/mmol for all CMR and subclinical CMR respectively and the 8.1 ng/mmol cutoff also identifies subclinical MCI (50%) and BTUB (20%).
This study validates urine CXCL10 as a sensitive biomarker of subclinical rejection. Other important subclinical phenotypes identified are MCI. We propose that CXCL10 may be useful for noninvasive screening of allograft inflammation in children.
To cite this abstract in AMA style:
Blydt-Hansen T, Gibson I, Dufault B, Ho J. Validation of Urine CXCL10 for Diagnosis of Subclinical and Clinical Rejection in Pediatric Kidney Transplants [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/validation-of-urine-cxcl10-for-diagnosis-of-subclinical-and-clinical-rejection-in-pediatric-kidney-transplants/. Accessed February 28, 2026.« Back to 2013 American Transplant Congress
