Use of HCV Ab+/NAT- Donors in HCV Naïve Renal Transplant Recipients
1Univeristy of Cincinnati Medical Center, Cincinnati, OH
2The Christ Hospital, Cincinnati, OH.
Meeting: 2018 American Transplant Congress
Abstract number: 111
Keywords: Donors, Hepatitis C, Kidney transplantation, marginal
Session Information
Session Name: Concurrent Session: Kidney Donor Selection / Management Issues - 1
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:42pm-4:54pm
Presentation Time: 4:42pm-4:54pm
Location: Room 6E
The continued organ shortage in renal transplantation (RT) has stimulated use of organs from increased-risk donors to expand the donor pool. The rate of denovo Hepatitis C (HCV) infection from the use of HCV antibody (HCVAb) positive and HCV nucleic acid test (HCVNAT) negative kidney allografts is unknown. Study aim was to prospectively analyze RT outcomes from HCVAb+/NAT- donors to HCV naïve recipients under T-cell depleting early steroid withdrawal (ESW) immunosuppression (IS).
Methods: HCV naïve RT recipients transplanted with a HCVAb+/NAT- allograft (Dec 2015-Nov 2017) were prospectively reviewed. Donor/recipient characteristics, IS strategies, and clinical outcomes (allograft function, graft/patient survival, and de novo HCV infection) were collected. Per center monitoring guidelines, recipients underwent HCV PCR testing (Roche COBAS® Ampliprep TNAI/ TaqMan® 48 RUO Assay; limit of detection 15 IU/ml) at 3 months post-RT or sooner if clinically indicated.
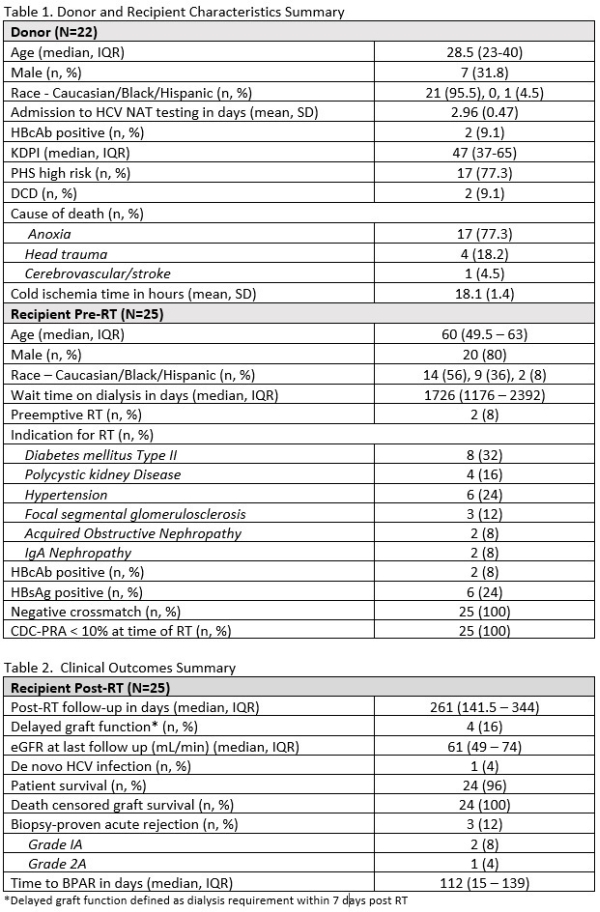
Results: Kidneys from 32 HCVAb+/NAT- donors were transplanted to 43 HCVAb- recipients at 2 centers. Analysis includes 25 recipients (allografts from 22 donors) with 3-month follow-up. Table 1 summarizes donor/recipient characteristics. HCVNAT testing within the eclipse period occurred in 95% of donors. IS received by 96% included anti-thymocyte globulin, tacrolimus, and 7-day ESW. Rate of de novo HCV infection is 4% (1/25). HCV viremia at 92 days post-RT in a 61 year old male transplanted for IgA nephropathy; initiation of anti-viral therapy (glecaprevir/pibrentasvir for 12 weeks) is underway. One death at 261 days post-RT due to unknown causes (autopsy not performed) was determined not related to HCV or donor factors. Table 2 summarizes clinical outcomes.
Conclusion: This represents the largest, prospective series of HCVAb+/NAT- donors transplanted to HCV naive recipients with a 4% rate of HCV transmission under T-cell depleting ESW IS. Our experience demonstrates an effective strategy to expand the donor pool, decrease waitlist times, and decrease discard rates of HCV positive kidneys.
CITATION INFORMATION: Dao A., Cuffy M., Kaiser T., Loethen A., Cafardi J., Shields A., Cardi M., Alloway R., Govil A., Sherman K., Shah S., Woodle E. Use of HCV Ab+/NAT- Donors in HCV Naïve Renal Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Dao A, Cuffy M, Kaiser T, Loethen A, Cafardi J, Shields A, Cardi M, Alloway R, Govil A, Sherman K, Shah S, Woodle E. Use of HCV Ab+/NAT- Donors in HCV Naïve Renal Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/use-of-hcv-ab-nat-donors-in-hcv-nave-renal-transplant-recipients/. Accessed February 22, 2026.« Back to 2018 American Transplant Congress