Use of Donor Blood Expedites HCV Genotyping and Allows Earlier DAA Initiation for Recipients of HCV+ Kidneys
B. Lonze, N. Ali, R. Montgomery, Z. Stewart Lewis
Tranplant Institute, NYU Langone Health, New York, NY
Meeting: 2021 American Transplant Congress
Abstract number: 365
Keywords: Cadaveric organs, Hepatitis C, Kidney transplantation
Topic: Clinical Science » Infectious Disease » Non-Organ Specific: Viral Hepatitis
Session Information
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:35pm-6:40pm
Presentation Time: 6:35pm-6:40pm
Location: Virtual
*Purpose: Utilization of HCV viremic donor kidneys for transplant into HCV naïve recipients has become more widespread, yet best practices governing the initiation, timing or duration of direct acting antiviral (DAA) therapy are lacking. Most published series describe DAA initiation weeks to months after transplant. However, fibrosing cholestatic hepatitis has been reported with delayed DAA initiation. Herein we report our center practice utilizing donor blood for HCV genotyping to expedite DAA insurance approval and minimize the duration of recipient viremia.
*Methods: Patients received education and DAA insurance benefits were ensured prior to listing for HCV+ organs. At the time of transplant, donor blood accompanying the kidney was used for HCV genotyping. Results were received within one week of transplant. Recipients were screened for HCV RNA by POD#4, and weekly for 12 weeks. Insurance authorization for DAA coverage was sought after both recipient viremia and donor HCV genotyping resulted. In 3 cases, donor viral load was insufficient for genotyping, and these recipients were genotyped once viremic.
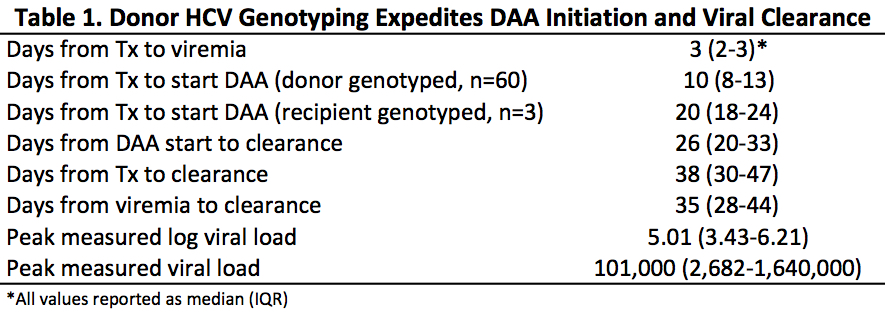
*Results: 80 hepatitis C naïve patients received hepatitis C positive donor kidneys between July, 2018 and October, 2020. 17 donors were HCV Ab+/NAT- and 63 donors were HCV Ab+/NAT+. All recipients of NAT+ donor organs became viremic; 89% were genotype 1a or 3. The median time to DAA initiation was 10 days for cases with donor genotyping (IQR 8-13). In contrast, the median time to DAA initiation was 20 days for the 3 cases with recipient genotyping (IQR 18-24). Median time from transplant to clearance of HCV viremia was 38 days (IQR 30-47) (Table 1). SVR12 was achieved in all patients, and no cases of fibrosing cholestatic hepatitis have been observed. There were 2 needlestick exposures of patient family members, though no HCV transmission occurred.
*Conclusions: Early HCV genotyping using donor blood results in expedited initiation of DAA therapy for recipients of HCV+ kidneys. Compared to published reports, our patients are clearing viremia at the time that most other centers’ patients are initiating DAA therapy. Whether duration of viremia or peak viral load are associated with adverse allograft events such as acute rejection is not known. The advantages to a shortened duration of HCV viremia remain to be characterized, but may include a lower risk of fibrosing cholestatic hepatitis and lower risk of HCV exposure to family members and caregivers. Our practice of expedited genotyping using donor blood is immediately implementable at all centers performing these transplants.
To cite this abstract in AMA style:
Lonze B, Ali N, Montgomery R, Lewis ZStewart. Use of Donor Blood Expedites HCV Genotyping and Allows Earlier DAA Initiation for Recipients of HCV+ Kidneys [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/use-of-donor-blood-expedites-hcv-genotyping-and-allows-earlier-daa-initiation-for-recipients-of-hcv-kidneys/. Accessed February 24, 2026.« Back to 2021 American Transplant Congress