Twice Daily Telaprevir in Combination with Peginterferon Alfa-2a/Ribavirin in HCV Genotype 1 Liver Transplant Recipients: Interim Safety/Efficacy of the REFRESH Study
Columbia University, New York, NY
Carolinas Medical Center, Charlotte, NC
Northwestern University, Chicago, IL
Henry Ford Hospital, Detroit, MI
University of Michigan, Ann Arbor, MI
Mayo Clinic, Scottsdale, AZ
University of British Columbia, Vancouver, BC, Canada
Vertex Pharmaceuticals Incorporated, Cambridge, MA
Meeting: 2013 American Transplant Congress
Abstract number: 367
Background: REFRESH is a 2-part Phase 2b, open-label clinical study assessing safety, efficacy, and pharmacokinetics (PK) of telaprevir (T) in combination with peginterferon alfa-2a (P) and ribavirin (R) in noncirrhotic liver transplant (LT) patients (pts) with recurrent genotype 1 hepatitis C virus (HCV). We report interim efficacy and safety data for 23 initial pts.
Methods: Pts on stable cyclosporine (CsA,n=3) or tacrolimus (TAC,n=20) received T 1125 mg BID + P 180 ¯o;g/wk + R 600 mg/day (initial dose) for 12 wks followed by 36 wks PR. Initial post-T CsA or TAC dose was reduced ∼4- and ∼10-fold (min. dose 0.5 mg) from baseline, respectively. No lead-in used.
Results: Enrolled pts (n=23) were 43-64 years old, 87% male, 91% White, 78% genotype 1a, 83% IL28B non-CC genotype, and 78% treated with PR. Median baseline HCV RNA was 6.9 log10 IU/mL. Fibrosis stage: 22% F0/1, 57% F2, 22% F3. Through Wk 4, key adverse events: anemia (n=9, 1 Grade 4; 5 erythropoietin; 1 transfusion), rash (n=8, all mild), anorectal symptoms (n=3, 1 moderate), pruritus (n=3, 1 moderate), renal insufficiency (n=3, all mild/moderate, none with supratherapeutic TAC or CsA). T was discontinued in 1 pt with high TAC levels; TAC had not been held at T onset. Available data for on-treatment HCV RNA levels is below.
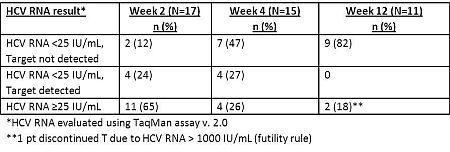
Conclusions: Interim analysis of a subset of post-transplant patients in ongoing REFRESH study suggests that telaprevir was used safely with CsA and TAC when used per protocol (TAC held at onset of telaprevir dosing). Preliminary data in these patients show that 47% (7/15) and 82% (9/11) of patients have undetectable HCV RNA at Wks 4 and 12, respectively.
Brown, R.: Other, Vertex Pharmaceuticals Incorporated, Consultant. Russo, M.: Grant/Research Support, Vertex Pharmaceuticals Incorporated, Speaker’s Bureau, Vertex Pharmaceuticals Incorporated. Levitsky, J.: Speaker’s Bureau, Vertex Pharmaceuticals Incorporated, Genentech. Brown, K.: Grant/Research Support, Exenenz, Speaker’s Bureau, Vertex Pharmaceuticals, Consultant, Merck, Consultant, Gilead, Consultant, Other, Salix, Consultant, BCBS Transplant Centers, Consultant. Fontana, R.: Grant/Research Support, Vertex Pharmaceuticals Incorporated, Gilead, Ocera, Other, Tibotec, Consulting, GSK, Consulting, Merck, Consulting. Vargas, H.: Grant/Research Support, Vertex Pharmaceuticals Incorporated, Janssen/Tibotec, BMS, Novartis, Merck, Gilead. Yoshida, E.: Other, Vertex Pharmaceuticals Incorporated, CME Honouraria, Vertex Pharmaceuticals Incorporated, Clinical Trial Investigator, Janssen Inc. Clinical Trial Investigator, Vertex Pharmaceuticals Incorporated, Board Meeting Honouraria. Bsharat, M.: Employee, Vertex Pharmaceuticals Incorporated, Stockholder, Vertex Pharmaceuticals Incorporated. Rubin, R.: Employee, Vertex Pharmaceuticals, Stockholder, Vertex Pharmaceuticals.
To cite this abstract in AMA style:
Brown R, Russo M, Levitsky J, Brown K, Fontana R, Vargas H, Yoshida E, Bsharat M, Rubin R. Twice Daily Telaprevir in Combination with Peginterferon Alfa-2a/Ribavirin in HCV Genotype 1 Liver Transplant Recipients: Interim Safety/Efficacy of the REFRESH Study [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/twice-daily-telaprevir-in-combination-with-peginterferon-alfa-2aribavirin-in-hcv-genotype-1-liver-transplant-recipients-interim-safetyefficacy-of-the-refresh-study/. Accessed March 3, 2026.« Back to 2013 American Transplant Congress
