Treatment of Chronic Active Antibody Mediated Rejection in Kidney Transplant Recipients is Associated with Increased Risk of Urinary Tract Infections and Pneumonia
University of Wisconsin, Madison, WI
Meeting: 2020 American Transplant Congress
Abstract number: B-070
Keywords: Infection, Kidney transplantation, Rejection
Session Information
Session Name: Poster Session B: Kidney Chronic Antibody Mediated Rejection
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Infections are a common cause of morbidity and mortality after kidney transplantation. Chronic active antibody mediated rejection (cABMR) is a leading cause of kidney graft failure. The risks of infection after treatment of cABMR have not been established.
*Methods: This was a single-center retrospective analysis of adult kidney transplant recipients diagnosed with cABMR between 01/01/2013 and 12/31/2016 and treated with pulse steroids, IVIG and/or rituximab. The control group consisted of patients who underwent a protocol biopsy for de-novo or rising donor specific antibodies (DSA) during this time period but did not have rejection. We examined the incidence of infectious complications including BKV, CMV, UTI and pneumonia between the two groups.
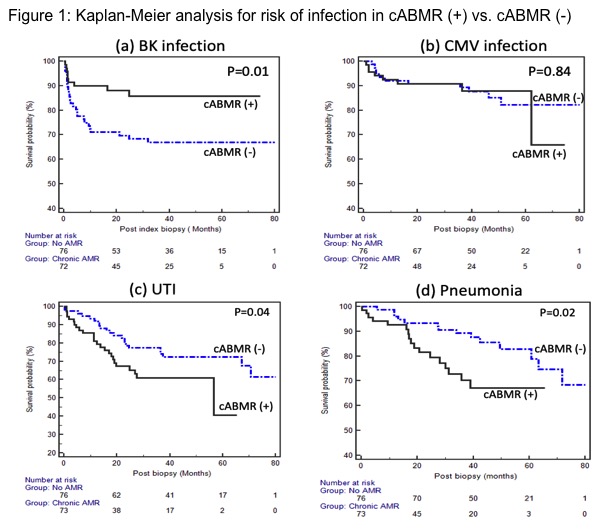
*Results: There were 73 patients in the cABMR (+) group and 76 patients in the cABMR (-) group. Patients with cABMR were younger, received more IL-2 induction and had higher serum creatinine and proteinuria levels at biopsy. Thirty-three patients (45%) received rituximab in addition to pulse steroids and IVIG. Mean follow up after biopsy was 42.2 ± 20.2 months. The mean time to biopsy for the cABMR (+) group was 114.1 ± 67.4 months vs. 32.6 ± 59.7 months in the cABMR (-) group. There were 59 total infectious complications in the cABMR (+) group compared to 72 in the cABMR (-) group (p=ns). Kaplan-Meier survival analyses demonstrated a significantly greater incidence of UTI and pneumonia in the cABMR (+) group, whereas BKV infection was more common in the cABMR (-) group. There was no difference in the incidence of CMV. (Figure 1) Multivariable Cox regression models were constructed to adjust for patient demographics, baseline characteristics, and induction therapy. These analyses confirmed an independent association between cABMR treatment and UTI (HR 2.59, 95% CI 1.32 to 5.05, p=0.005) and pneumonia (HR 2.68, 95% CI 1.21 to 5.93, p=0.01). However, rituximab was not independently associated with risk of BKV, CMV, UTI or pneumonia.
*Conclusions: Compared to patients without rejection, cABMR and its treatment with pulse steroids, rituximab and IVIG was associated with ~ 2.5 times greater risk of UTI and pneumonia. BKV infection was more common in the cABMR (-) group but this group of patients was earlier in their post-transplant course given shorter time from transplant to biopsy. Further studies are needed to help determine the independent role of these immunomodulatory agents in the incidence of infection after cABMR.
To cite this abstract in AMA style:
Joachim E, Parajuli S, Swanson K, Aziz F, Garg N, Mohamed M, Mandelbrot D, Djamali A. Treatment of Chronic Active Antibody Mediated Rejection in Kidney Transplant Recipients is Associated with Increased Risk of Urinary Tract Infections and Pneumonia [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/treatment-of-chronic-active-antibody-mediated-rejection-in-kidney-transplant-recipients-is-associated-with-increased-risk-of-urinary-tract-infections-and-pneumonia/. Accessed February 18, 2026.« Back to 2020 American Transplant Congress