Transplantation of Kidneys from Hepatitis C Infected Donors to Hepatitis C Negative Recipients
M. Z. Molnar, O. Cseprekal, M. Yazawa, M. Talwar, V. Balaraman, V. Mas, D. Maluf, R. Helmick, L. Campos, N. Nezakatgoo, C. Eymard, P. Horton, R. Verma, A. Jenkins, C. R. Handley, H. Snyder, C. Cummings, U. A. Agbim, B. Maliakkal, S. K. Satapathy, S. P. Nair, J. D. Eason
Methodist University Hospital Transplant Institute, Memphis, TN
Meeting: 2019 American Transplant Congress
Abstract number: 19
Keywords: Donors, unrelated, Hepatitis C, Kidney transplantation, Outcome
Session Information
Session Name: Concurrent Session: Kidney Donor Selection / Management Issues I
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:30pm-2:42pm
Presentation Time: 2:30pm-2:42pm
Location: Ballroom C
*Purpose: There are no data on the safety of kidney transplantation from hepatitis C (HCV) NAT positive donors to HCV naïve recipients. We report the preliminary data of 58 HCV negative recipients who received HCV NAT positive kidneys between March and October, 2018 in our center.
*Methods: All recipients received induction with Thymoglobulin 4.5 mg/kg and maintenance immunosuppression with tacrolimus, mycophenolic-acid and prednisolone. Treatment with a pan genotypic DAA regimen was offered to recipients at 1-3 months after complete recovery from the renal transplant and once HCV RNA was detected.
*Results: Tables 1 and 2 detail recipient and donor characteristics.
| Age, years (mean (SD)) | 52 (11) |
| Female (N (%)) | 21 (36) |
| Caucasians (N (%)) | 10 (17) |
| Time on dialysis, months (mean (SD)) | 51 (30-75) |
| Diabetes (N (%)) | 29 (50) |
| Peripheral vascular disease (N (%)) | 7 (12) |
| Coronary artery disease (N (%)) | 9 (16) |
| Medicare insured (N (%)) | 46 (79) |
| Age, years (mean (SD)) | 32 (5) |
| Female (N (%)) | 22 (38) |
| DCD (N (%)) | 6 (10) |
| Diabetes (N (%)) | 1 (2) |
| Hypertension (N (%)) | 4 (7) |
| Caucasians (N (%)) | 55 (95) |
| KDPI (mean (SD)) | 51 (15) |
| KDPI without HCV component (mean (SD)) | 24 (15) |
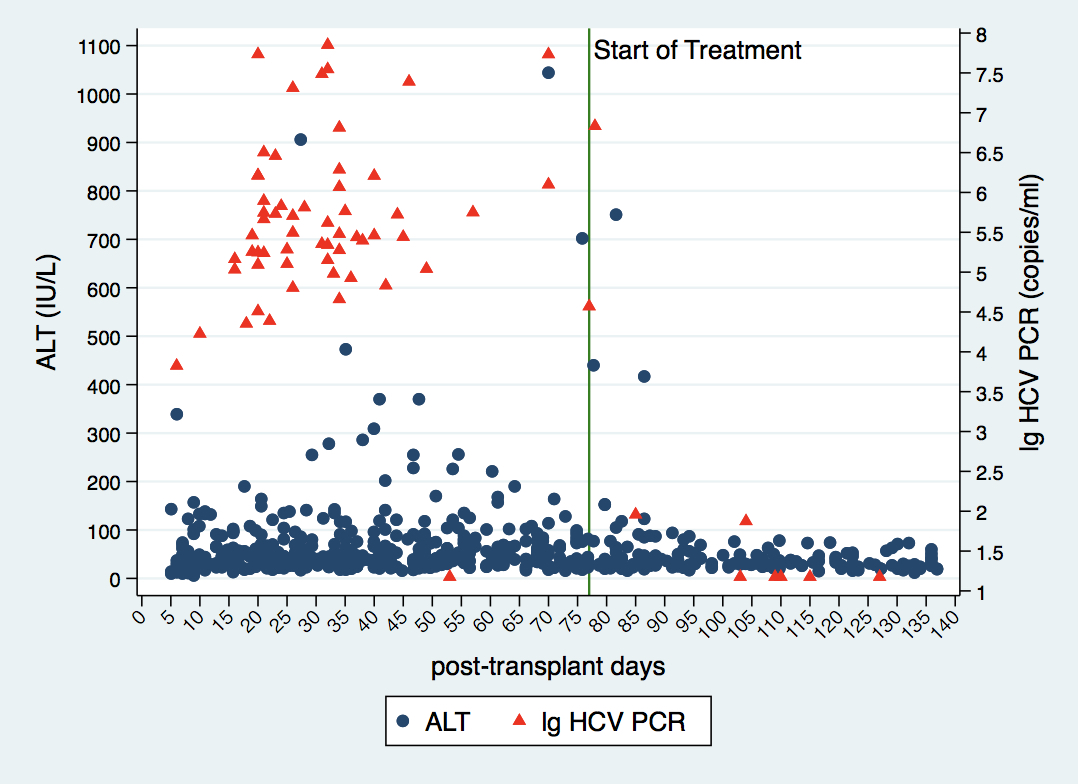
The median (IQR) time between transplant and treatment initiation was 77 (68-90) days. The time waiting for insurance approval was 17 (12-25) days. All 58 patients became viremic (genotype: 1a (N=38), 1b (N=1), 2 (N=3) and 3 (N=16)). This Figure shows ALT levels and virus copies before and after treatment.
At this time 53 recipients were started on treatment. Forty out of 41 became PCR negative at 4 weeks of treatment. Fourteen patients completed treatment and were negative at the end of treatment. Four recipients had delayed graft function and one developed cellular rejection. The estimated GFR at 4, 12, and 16 weeks was 56±16 ml/min, 58±17 ml/min, and 66±16 ml/min, respectively.
*Conclusions: Kidney transplantation from Hepatitis C positive donors to negative recipients is safe and effective, but further long-term data is needed from this and other cohorts outside clinical trials. Using HCV positive kidneys in naïve recipients provides life-saving benefit with negligible risk.
To cite this abstract in AMA style:
Molnar MZ, Cseprekal O, Yazawa M, Talwar M, Balaraman V, Mas V, Maluf D, Helmick R, Campos L, Nezakatgoo N, Eymard C, Horton P, Verma R, Jenkins A, Handley CR, Snyder H, Cummings C, Agbim UA, Maliakkal B, Satapathy SK, Nair SP, Eason JD. Transplantation of Kidneys from Hepatitis C Infected Donors to Hepatitis C Negative Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/transplantation-of-kidneys-from-hepatitis-c-infected-donors-to-hepatitis-c-negative-recipients/. Accessed February 21, 2026.« Back to 2019 American Transplant Congress