Transplant Certification and Quality Assessment: The Effect of Center Data Input Quality
Center for Abdominal Transplantation, Saint Louis University, St. Louis
Dartmouth College, Lebanon
University of Texas, San Antonio
Meeting: 2013 American Transplant Congress
Abstract number: A774
Each transplant program in the United States is required to submit self-reported data to OPTN/UNOS to calculate a center’s performance. Currently, under the Conditions of Participation by CMS, these data are used to support compliance with performance standards. In this study, we illustrate the benefits of utilizing process control charts to prospectively monitoring our center’s outcomes, as well as the benefits of implementing procedures that ensure the quality of publicly reported data.
Methods: In response to a CMS citation of our liver program and to better understand center performance, a system of process control charts was employed that capture current performance and project future SRTR report outcomes based on observed patterns. These charts display the observed adverse events (graft failures or deaths) over time compared to the risk adjusted, expected number of adverse events and signaling thresholds limits using the SRTR equations.
Results: Using these plots, we were able to project that our center would be flagged by CMS and given a Condition Level Citation after the next SRTR release (Figure A), likely resulting in a Systems Improvement Agreement (SIA). This lead us to perform a complete review of data used by the SRTR for risk adjustment. Several incorrect data entry policies were found and extensive data corrections were reentered into UNet. Correction of this data led to a substantial improvement in reported center performance such that a CMS citation is unlikely and may be impossible (Figure B).
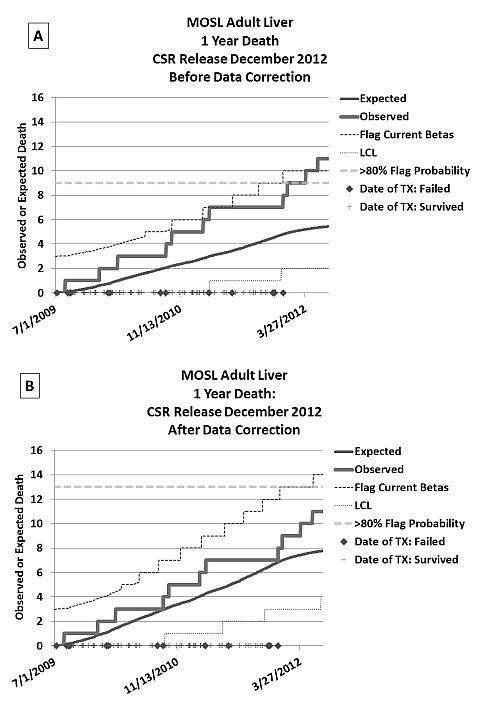
Conclusion: In the current competitive environment, it is imperative for each program, and its care providers, to accurately collect and submit data, appreciate how these data are used in risk adjustments for their specific program, document the potential sources of unrecognized risk in their patient population and organ supply, and prospectively assess their own performance continuously. We speculate that data entry error alone may be the root cause of many CMS citations and SIAs.
Lentine, K.: Stockholder, XynManagement, LLC. Axelrod, D.: Stockholder, XynManagement, LLC. Schnitzler, M.: Stockholder, XynManagement, LLC.
To cite this abstract in AMA style:
Tuttle-Newhall J, Lentine K, Axelrod D, Ware L, Milton J, Schnitzler M. Transplant Certification and Quality Assessment: The Effect of Center Data Input Quality [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/transplant-certification-and-quality-assessment-the-effect-of-center-data-input-quality/. Accessed February 23, 2026.« Back to 2013 American Transplant Congress
