Timing, Duration, and Source of Hepatitis C Virus (HCV) Antibodies (Ab) among HCV-Negative Recipients of an Organ from an HCV-Infected Donor
P. Porrett1, P. Reese1, E. Blumberg1, P. Abt1, R. Bloom1, K. Reddy1, V. Holzmayer2, K. Coller2, M. Kuhns2, G. Cloherty2, D. Goldberg1
1University of Pennsylvania, Philadelphia, PA, 2Abbott Labs, Chicago, IL
Meeting: 2019 American Transplant Congress
Abstract number: D236
Keywords: Antibodies, Hepatitis C
Session Information
Session Name: Poster Session D: Non-Organ Specific: Viral Hepatitis
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: The opioid epidemic has increased the number of deceased donors with active (Ab+/NAT+) or prior (Ab+/NAT-) HCV infection. Increased utilization of these donor organs has led to reports of previously HCV Ab-negative organ recipients testing positive for anti-HCV Ab. Yet it is unknown if the Ab was donor- and/or recipient-derived, and the implications of such transmissions.
*Methods: We banked sera from HCV Ab-negative kidney or heart transplant (KT, HT) recipients of HCV NAT+ organs [THINKER (NCT02743897) and USHER (NCT031467), respectively] as early as day 1 post-transplant. Ab testing was performed using the ARCHITECT Anti-HCV assay (Abbott laboratories), followed by confirmatory testing of repeatedly reactive samples (INNO-LIA HCV Score assay; Fujirebio). Subsequent anti-HCV IgM and IgG research-only assays were developed to distinguish HCV IgG vs IgM Ab specificity.
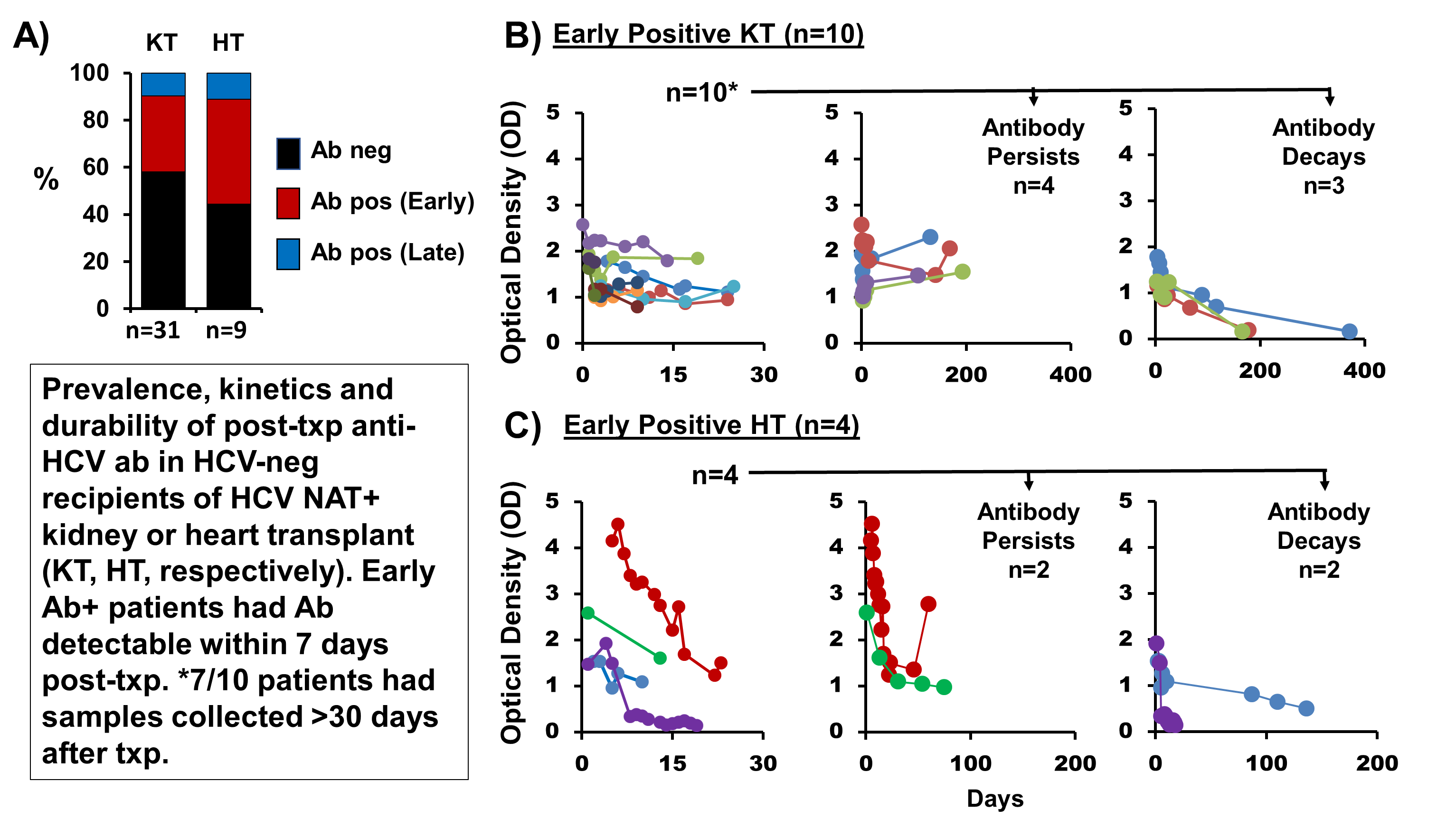
*Results: From 6/1/16-5/23/18, 40 HCV Ab-negative patients received a KT in the THINKER trial (31 had samples for analysis from 1-371 days post-KT; all received thymoglobulin), and 10 received a HT in the USHER trial (9 had samples for analysis from 1-163 days post-HT; all received basiliximab). During follow up of the 40 subjects with available samples, 18 (45%) had detectable anti-HCV Ab (≥1.0 optical density): 42% of KT recipients (13/31) and 56% of HT recipients (5/9). Ab results were concordant for all recipients of a given donor (5 recipients shared 2 donors) The timing of anti-HCV Ab in the first 30 days post-transplant are shown [Figure]. In KT recipients, early Ab persisted for >30 days at a relatively stable level before declining; in HT recipients, early Ab level decreased quickly in all patients and dropped below detection in half of patients within two weeks of HT. There were 81 unique samples that tested positive for anti-HCV Ab and 100% were IgG. There was no difference in donor or recipient viral loads among those who did vs did not develop an HCV Ab (p>0.2).
*Conclusions: Anti-HCV Ab is seen very early in nearly half of HCV-negative recipients of an HCV NAT+ organ. The mature IgG isotype of this Ab as well as the kinetics of its appearance and durability suggest the Ab is donor-derived and likely produced by a cellular source. Future studies are needed to identify whether donor-derived plasma cells are the source of Ab. A positive anti-HCV Ab in an organ recipient from an HCV Ab+ donor should be interpreted with caution as donor-derived Ab may be misinterpreted as evidence of disease transmission.
To cite this abstract in AMA style:
Porrett P, Reese P, Blumberg E, Abt P, Bloom R, Reddy K, Holzmayer V, Coller K, Kuhns M, Cloherty G, Goldberg D. Timing, Duration, and Source of Hepatitis C Virus (HCV) Antibodies (Ab) among HCV-Negative Recipients of an Organ from an HCV-Infected Donor [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/timing-duration-and-source-of-hepatitis-c-virus-hcv-antibodies-ab-among-hcv-negative-recipients-of-an-organ-from-an-hcv-infected-donor/. Accessed March 6, 2026.« Back to 2019 American Transplant Congress