The Safety and Efficacy of 6-Month Steroid Reduction Strategy after Kidney Transplantation: A Prospective Cohort Study
Department of Surgery, Ajou University Medical School, Suwon, Korea, Republic of
Meeting: 2020 American Transplant Congress
Abstract number: C-116
Keywords: Glucocortocoids, Immunosuppression, Post-transplant diabetes, Safety
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: In kidney transplantation, immunosuppressive regimens almost always contain corticosteroids for preventing acute rejection and graft loss. However, long-term maintenance of corticosteroids has been associated with co-morbidity, with adverse effects including weight gain, hyperlipidemia, high blood pressure, impaired glucose metabolism and osteoporosis. Although several studies have been conducted to evaluate the efficacy of corticosteroid withdrawal after kidney transplantation, the optimal timing of corticosteroid withdrawal is still remained as a considerable debate because of increased risk of allograft loss and acute rejection. The aim of this study was to evaluate the safety and efficacy of steroid reduction strategy after 6-month post-kidney transplantation.
*Methods: A prospective, multicenter, open-label, single arm study was conducted at 12 transplant centers in Korea. A total sample size was 179 and among these patient, 6-month steroid reduction strategy applied to 65 patients who satisfied the inclusion criteria (no biopsy-proven acute rejection (BPAR) in the first 6-month, no proteinuria, no elevated serum creatinine). This 1-year follow-up study assessed the safety and efficacy of 6-month steroid reduction strategy.
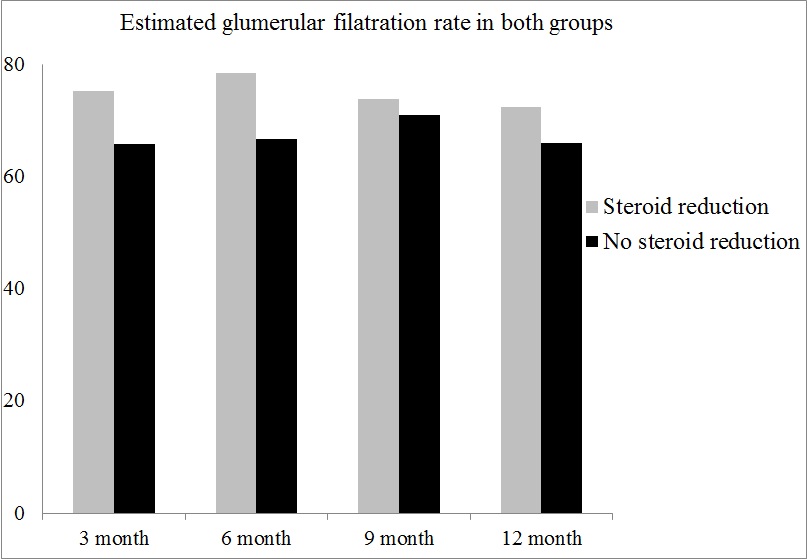
*Results: The mean estimated glomerular filtration rates at 1-year post-transplantation were 65.91 ± 19.62 mL/min/1.73m2 in no steroid reduction group and 72.26 ± 19.07 mL/min/1.73m2 in steroid reduction group (P=0.037). In the time interval from 6-month to 1-year after transplantation, the incidences of BPAR were 4 (3.5%) in no steroid reduction group and 0 in steroid reduction group. No graft loss and patient death was observed in both groups. There was a small but not statistical difference in HbA1c levels at 1-year between two groups (6.23±1.11 vs 5.92±1.14, P=0.074). In steroid reduction group, there was no incidence of new onset diabetes mellitus after transplantation (NODAT) during 6-month to 1-year post-transplantation.
*Conclusions: In this study, our findings suggest that the strategy of 6-month after steroid reduction in kidney transplantation is safe and effective to the patient with no adverse course in the first 6-month post-transplantation. Additionally, it is expected that a glucose metabolism is improved by steroid reduction strategy without an increase in acute rejection and graft loss rates comparing to steroid maintenance regimen.
To cite this abstract in AMA style:
Bang J, Oh C, Lee S. The Safety and Efficacy of 6-Month Steroid Reduction Strategy after Kidney Transplantation: A Prospective Cohort Study [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/the-safety-and-efficacy-of-6-month-steroid-reduction-strategy-after-kidney-transplantation-a-prospective-cohort-study/. Accessed March 8, 2026.« Back to 2020 American Transplant Congress