The Role of Complement-Split Products as Biomarkers for Acute Antibody-Mediated Rejection of Kidney Allografts
1University of Cincinnati, Cincinnati, OH, 2All India Institute of Medical Sciences, New Delhi, India, 3University of Colorado, Aurora, CO
Meeting: 2020 American Transplant Congress
Abstract number: B-315
Keywords: Antibodies, Kidney transplantation, Rejection
Session Information
Session Name: Poster Session B: Biomarkers, Immune Assessment and Clinical Outcomes
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Acute antibody-mediated rejection (AMR) is mediated by the activation of the classical complement system in addition to non-complement dependent inflammatory pathways. Complement fixation by donor specific antibodies (DSA) leads to cleavage of the complement proteins C4, C3 and C5 to produce multiple complement-split products (CSP) and the end-effector membrane attack complex, C5b-9. In this study, we investigate CSP as potential biomarkers for AMR.
*Methods: In an IRB-approved, prospective, controlled study, CSP levels were measured in blood and urine samples from consecutive kidney transplant (KTx) recipients with biopsy-proven AMR (n=10), acute cellular rejection (ACR) (n=5) or no rejection (NR) (n=5). Patients with mixed rejection were included under the AMR arm. After obtaining informed consent, samples were collected at the time of biopsy (day 0), and days 15 (end of rejection treatment) and 30 post-biopsy for AMR and ACR patients. ELISA was used to measure C5a, C4d and soluble C5b-9 (sC5b-9) concentrations in blood (EDTA plasma) and urine, in addition to Bb concentration in blood only. KTx histopathology was evaluated using the Banff 2013 classification. Rejection treatment and follow up was performed as per standard of care.
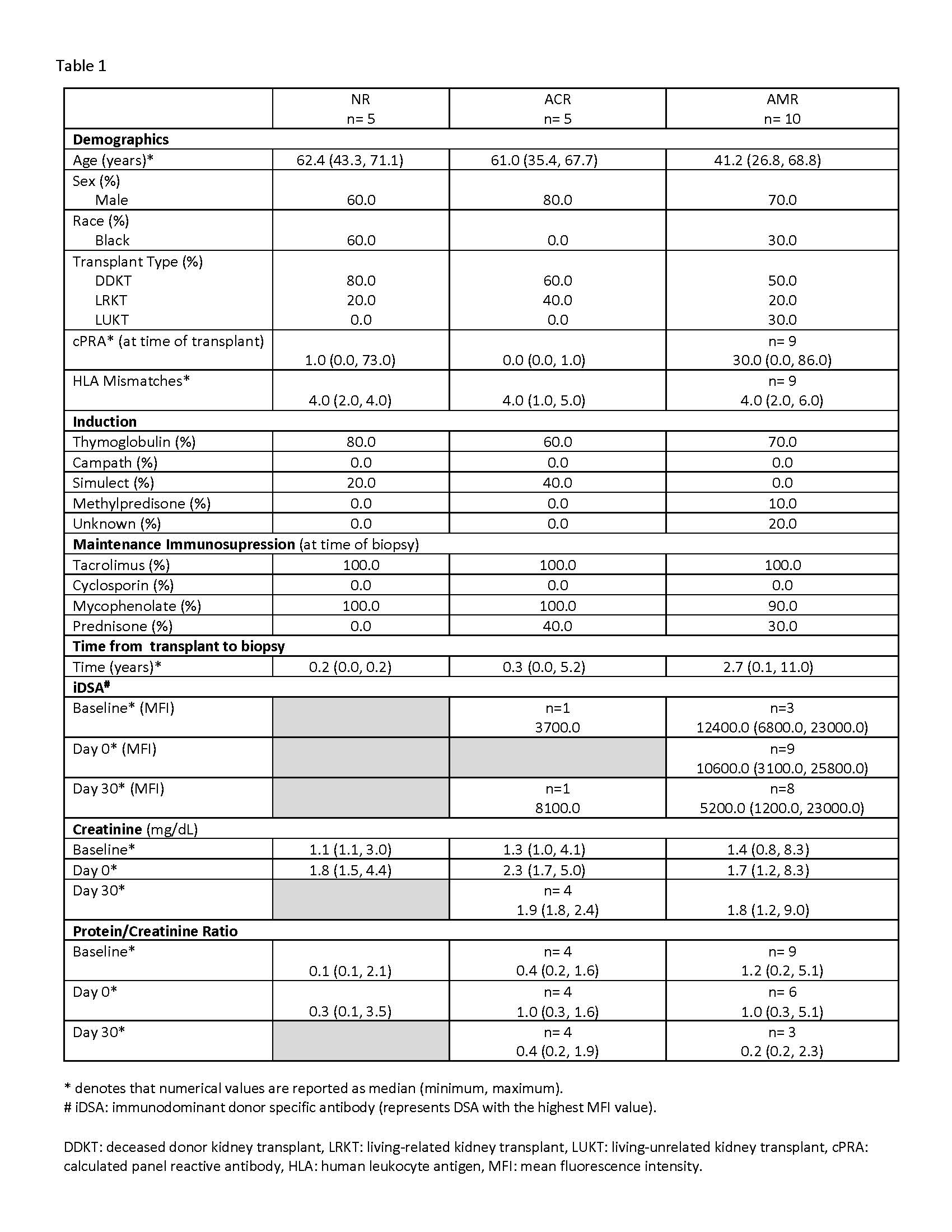
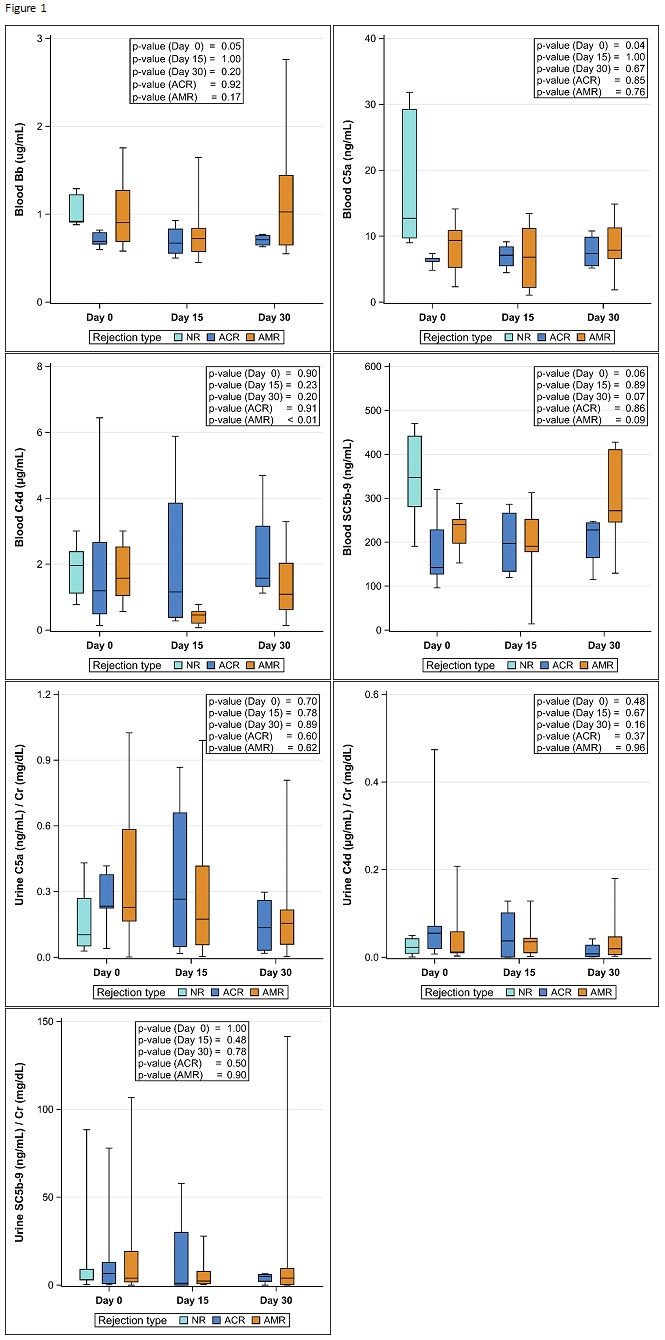
*Results: Baseline characteristics and results are shown in Table 1 and Figure 1.
*Conclusions: Blood and urine CSP levels adjusted to urine creatinine (Cr) were not elevated in AMR compared to NR and ACR arms. There was significant variability in CSP concentrations within each of the three study groups. Our study does not support the utility of CSP as surrogate markers of AMR, however it is limited by the small sample size and larger studies may be warranted.
To cite this abstract in AMA style:
Jawdeh BGAbu, Campos-Naciff B, Harrison K, Meganathan K, Kumar A, Anand M, Govil A, Woodle ES, Dixon BP. The Role of Complement-Split Products as Biomarkers for Acute Antibody-Mediated Rejection of Kidney Allografts [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/the-role-of-complement-split-products-as-biomarkers-for-acute-antibody-mediated-rejection-of-kidney-allografts/. Accessed February 26, 2026.« Back to 2020 American Transplant Congress