The Impact of De Novo Dose-Adjustment Strategies with Tacrolimus XR (LCP-Tac) in Kidney Transplant Recipients
Medical University of South Carolina, Charleston, SC
Meeting: 2021 American Transplant Congress
Abstract number: 419
Keywords: FK506, Immunosuppression, Pharmacokinetics, Rejection
Topic: Clinical Science » Pharmacy » Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Information
Session Name: Pharmacokinetics, Pharmacogenetics, and Drug Interactions, Oh My!
Session Type: Poster Video Chat
Date: Sunday, June 6, 2021
Session Time: 7:30pm-8:30pm
 Presentation Time: 7:40pm-7:50pm
Presentation Time: 7:40pm-7:50pm
Location: Virtual
*Purpose: LCP-Tac is a once daily formulation of tacrolimus indicated for the prophylaxis of rejection in de novo kidney transplant recipients. It provides less variable bioavailability, but high intra- and inter-patient variability remain a challenge. We sought to determine the impact of baseline characteristics and dose-adjustment strategies on achieving target levels of LCP-Tac in adult kidney transplant recipients.
*Methods: This was a retrospective longitudinal study of adult kidney recipients transplanted between May and September 2020 who received de novo LCP-Tac. The primary endpoint was time to first therapeutic level (7-12 ng/mL). Secondary endpoints included time to stable level and time in therapeutic range (TTR) in the first month. Categorical data were analyzed with chi square or fisher’s exact. Continuous data were assessed using one-way ANOVA. Multivariable linear regression analysis of the primary endpoint included variables that were statistically associated in univariable analyses.
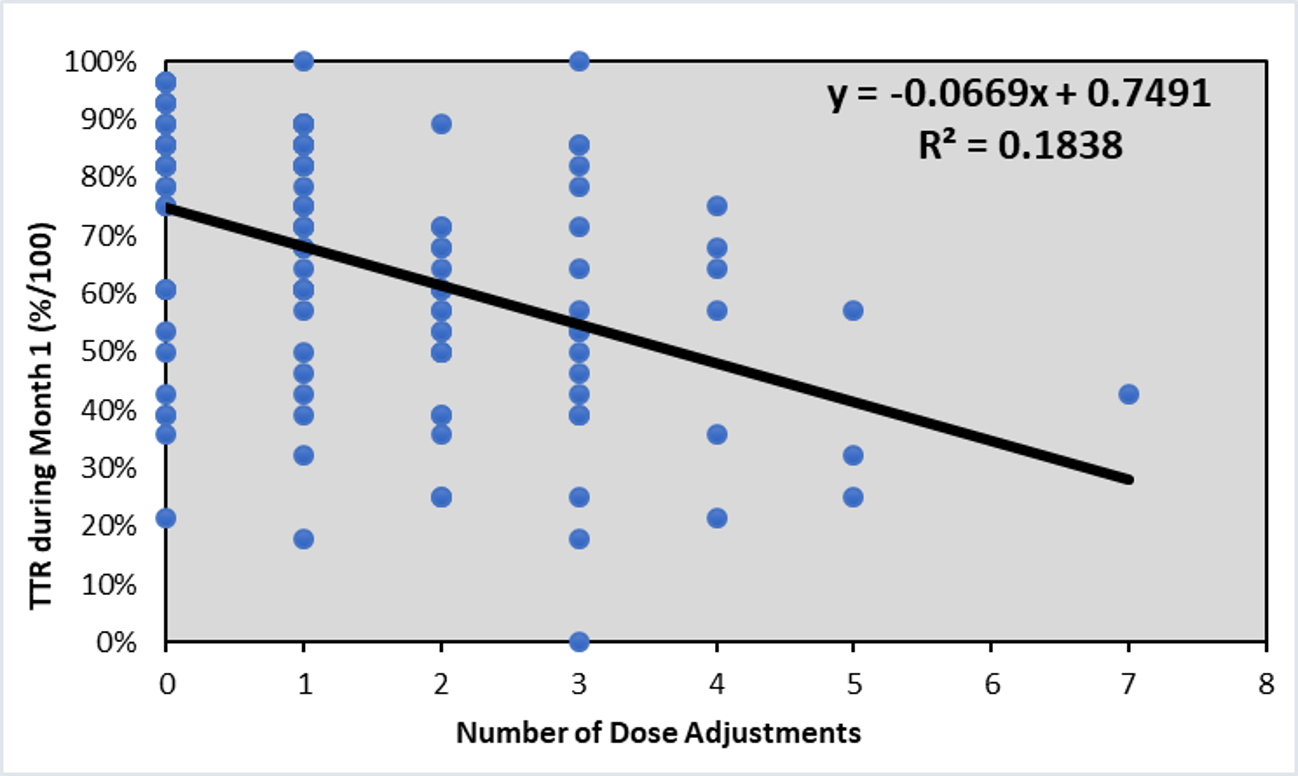
*Results: A total of 122 patients were included. Median time to first therapeutic level was 5 days. We assessed baseline and peri-operative factors; none of which impacted the primary endpoint (Table 1). Median time to stable level was 13 days. Eighteen patients never achieved a stable level during 1-month follow-up. TTR in the first month was 65% ± 22%. In the multivariable analysis, over-conservative dose adjustments significantly impacted TTR; for every dose adjustment ≥ 20% lower than expected using linear kinetics, the TTR decreased by 6.3% (95% CI 3.5% to 9.1%; p<0.01).
*Conclusions: Most patients given de novo LCP-Tac achieve a therapeutic level in the first week post-transplant, which does not appear to be influenced by baseline or post-op characteristics. TTR was significantly impacted by the number of dose adjustments lower than expected. This study emphasizes the importance of avoiding under-adjusting doses early after transplant with de novo LCP-Tac use.
Table 1. Multivariable model of time to first therapeutic level
Figure 1. Linear regression for number of dose adjustments that were at least 20% below expected and the TTR during the first month post-transplant
To cite this abstract in AMA style:
Carcella T, Bartlett F, Patel N, Rohan V, Taber D. The Impact of De Novo Dose-Adjustment Strategies with Tacrolimus XR (LCP-Tac) in Kidney Transplant Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/the-impact-of-de-novo-dose-adjustment-strategies-with-tacrolimus-xr-lcp-tac-in-kidney-transplant-recipients/. Accessed February 20, 2026.« Back to 2021 American Transplant Congress