Testing Deceased Organ Donors for Infections: An Organ Procurement Organization (OPO) Survey
1University of Massachusetts Medical School, Worcester, MA, 2UCSF, San Francisco, CA, 3Uniformed Services University School of Medicine, Bethesda, MD, 4Northwestern University, Chicago, IL
Meeting: 2020 American Transplant Congress
Abstract number: 167
Session Information
Session Name: Donor Derived Infections
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:39pm-3:51pm
Presentation Time: 3:39pm-3:51pm
Location: Virtual
*Purpose: We surveyed the 58 U.S. OPOs to update our knowledge about the current methods of infectious disease testing in deceased organ donors.
*Methods: An IRB approved survey was sent out using REDCap. AOPO also approved our project. Two reminder emails were sent. Data collection is ongoing.
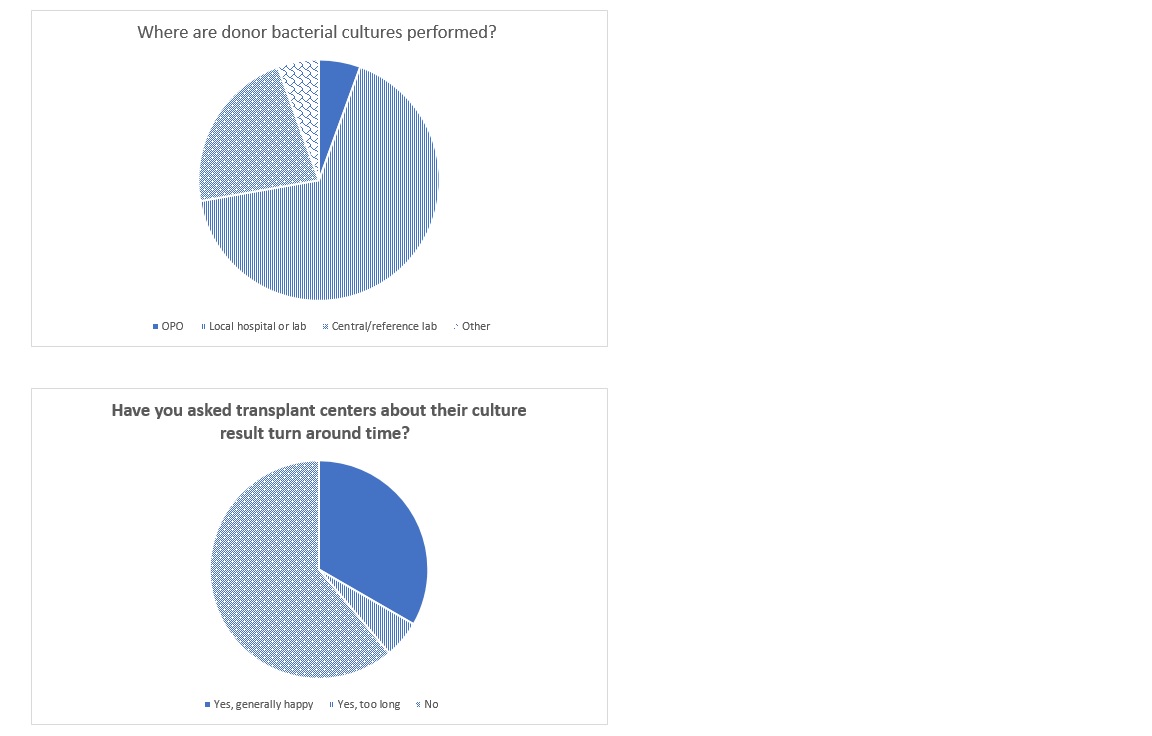
*Results: 20/58 (34%) OPO medical directors opened the survey and 18 provided responses to most of the questions. 9/11 UNOS regions are represented. 15/18 respondents (83%) state they consult an Infectious Diseases physician when needed. 9/18 respondents (50%) test for West Nile virus (WNV), the majority using nucleic acid testing (NAT) alone; of those testing, 8/9 (89%) test for WNV year-round. 6/18 (33%) respondents test for Strongyloides infection; of those testing, 3/6 (50%) test all donors and 3/6 (50%) test based on risk factors. 7/18 (39%) test for Chagas; 67% test only in patients with risk factors. One respondent tests all donors for Coccidioides. 2 respondents test for Zika virus. For syphilis testing, 13/17 (76%) respondents use RPR and 10/17 (59%) confirm positive results with a treponemal assay. No respondents test for HTLV. All respondents perform prospective NAT for HIV, hepatitis B and hepatitis C on all donors, with the majority using a transcription mediated amplification platform. Most respondents have not discussed turn around time of donor bacterial cultures with transplant centers, and most use a local hospital or lab to perform these cultures (Figure 1).
*Conclusions: Our survey reveals that since the last survey in 2012, testing all deceased organ donors for HIV, hepatitis B, hepatitis C with NAT has increased to 100% from 70% of OPOs responding. However, testing for other infections remains variable.
To cite this abstract in AMA style:
Theodoropoulos NM, Chin-Hong P, Greenwald M, Ison MG. Testing Deceased Organ Donors for Infections: An Organ Procurement Organization (OPO) Survey [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/testing-deceased-organ-donors-for-infections-an-organ-procurement-organization-opo-survey/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress