Tacrolimus Dosing, Trough Concentrations and Dose Adjustments Compared Between Two Generic Formulations in Adult Kidney Transplant Recipients.
MUSC, Charleston, SC.
Meeting: 2016 American Transplant Congress
Abstract number: D129
Keywords: Immunosuppression, Kidney transplantation, Pharmacokinetics
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Novel Agents
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Objective: The aim of this study was to assess if changing between two different formulations of generic tacrolimus ([tac] Mylan® and Dr. Reddy's Lab®) impacts trough concentration, total dose, and number of dose adjustments and levels during the first 30 days post-transplant in adult kidney transplant patients.
Methods: Single center retrospective cohort study comparing two different time periods: 4/1/09 to 7/31/11 (n=180; Mylan®, Cohort 1) vs 5/1/14 to 8/31/14 (n=50; Dr. Reddy's Lab®, Cohort 2). Daily doses, tac troughs and adjustments were recorded for 30-days post-transplant, along with baseline characteristics. All received tac, MMF and prednisone with induction therapy. Univariate and multivariable statistical analyses were utilized to compare groups.
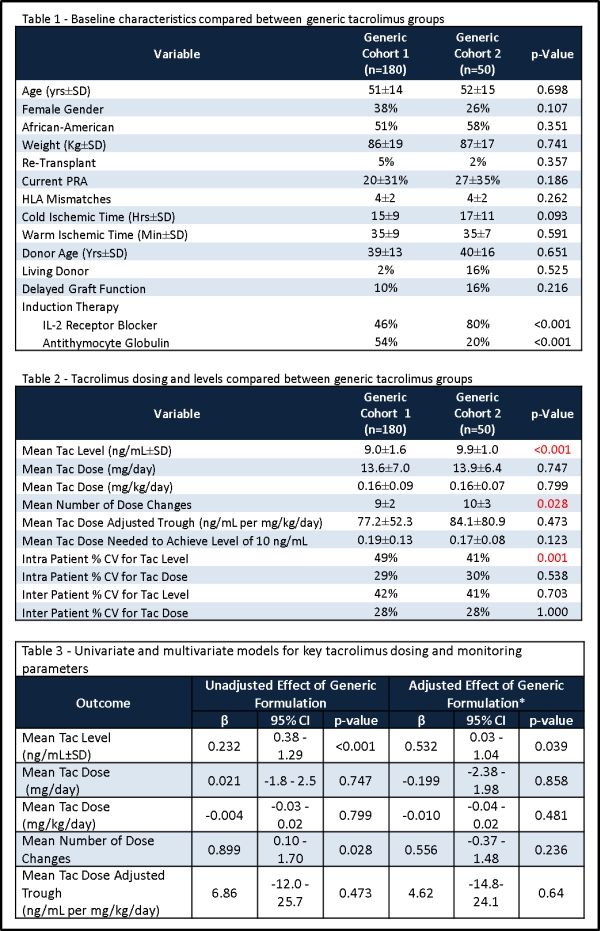
Results: 230 patients were included. The groups were comparable with regards to baseline characteristics, except that the Dr. Reddy's Lab cohort tended to have longer cold times and utilize more IL-2 induction (Table 1). In the univariate analyses, the mean tac trough was higher in Cohort 2, as was the number of dose adjustments. Other tac PK variables were similar between groups (Table 2). In multivariable analyses, after adjusting for known confounders, only the tac trough remained different (Table 3).
Conclusion: These data demonstrate that PK differences in generic formulations of tac are likely to reflect trends in prescribing practices and other confounders more so than actual generic formulation differences.
CITATION INFORMATION: Taber D, Phan V, Wilton A, Pilch N, Meadows H, Fleming J, Mardis C, Mardis A, Bratton C, Chavin K, McGillicuddy J, Nadig S, Baliga P. Tacrolimus Dosing, Trough Concentrations and Dose Adjustments Compared Between Two Generic Formulations in Adult Kidney Transplant Recipients. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Taber D, Phan V, Wilton A, Pilch N, Meadows H, Fleming J, Mardis C, Mardis A, Bratton C, Chavin K, McGillicuddy J, Nadig S, Baliga P. Tacrolimus Dosing, Trough Concentrations and Dose Adjustments Compared Between Two Generic Formulations in Adult Kidney Transplant Recipients. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/tacrolimus-dosing-trough-concentrations-and-dose-adjustments-compared-between-two-generic-formulations-in-adult-kidney-transplant-recipients/. Accessed February 23, 2026.« Back to 2016 American Transplant Congress
