Surveillance of Donor-Derived Cell-Free DNA Throughout Treatment of Acute Rejection in Pediatric Renal Transplant Recipients
1Pharmacotherapy Education and Research Center, UT Health San Antonio, San Antonio, TX, 2CareDx, Inc., Brisbane, CA, 3Long School of Medicine, UT Health San Antonio, San Antonio, TX
Meeting: 2022 American Transplant Congress
Abstract number: 373
Keywords: Kidney, Pediatric, Rejection
Topic: Basic Science » Basic Science » 02 - Acute Rejection
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:10pm-6:20pm
Presentation Time: 6:10pm-6:20pm
Location: Hynes Room 309
*Purpose: Donor-derived cell-free DNA (dd-cfDNA) is a dynamic plasma biomarker for allograft rejection with limited data in pediatric renal transplant recipients and few reports describing dd-cfDNA behavior throughout an episode of rejection from time of biopsy to therapy completion. This study presents dd-cfDNA changes prior to BPAR and post-treatment of rejection.
*Methods: Dd-cfDNA (AlloSure®) levels were drawn prior to and at time of biopsy, and prior to each treatment throughout the BPAR therapy course. Surveillance and for-cause biopsies were performed with dd-cfDNA levels collected per institutional protocol.
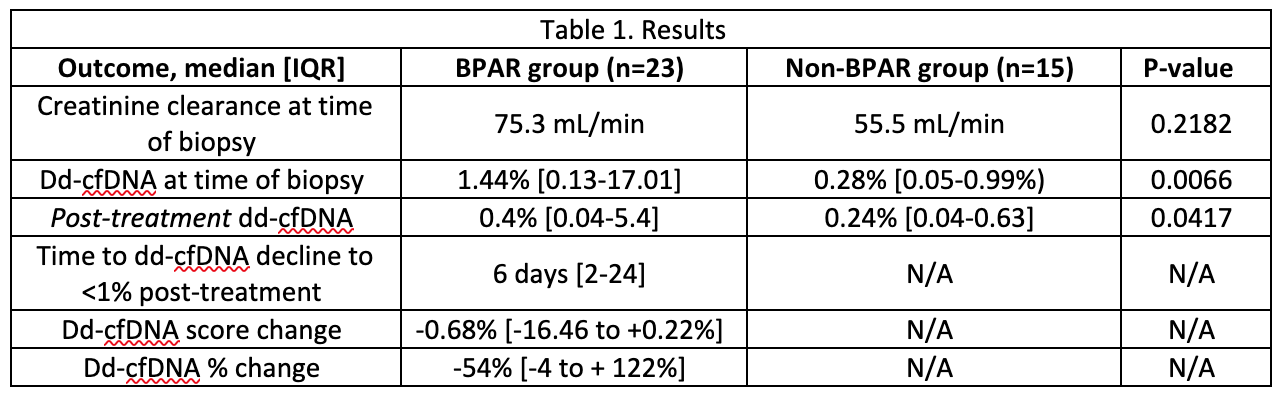
*Results: Forty two patients had dd-cfDNA testing and biopsy with 13 patients having more than 1 biopsy (56 biopsies total, 37 for-cause, 19 surveillance). There were 32 BPAR episodes in 23 patients [21 T-cell mediated rejection (TCMR) or borderline, 11 antibody-mediated rejection (AMR) or mixed]. In the entire cohort, receiver operator curves for dd-cfDNA predicted BPAR (AUC 0.795) and de novo DSA (AUC 0.75). Complete data was available for 23 biopsies with BPAR, and 15 biopsies without (Table 1). In the BPAR cohort, 13 of the 23 BPAR episodes had dd-cfDNA >1% at biopsy, and 8 of those 13 patients had dd-cfDNA decline to <1% after treatment. The median change in patients with TCMR was not significantly different compared to those with AMR [-0.2% (-16.46 to +0.22%) vs -0.94% (-2.15 to -0.04%), P=0.7763]. Creatinine clearance (CrCl) in the BPAR group was not significantly different pre- and post-treatment (median 55.5 vs 59.1 mL/min, P=0.8009). Two patients had repeat biopsies for persistently high cfDNA post-rejection treatment. One had persistent TCMR, and dd-cfDNA levels decreased to <1% after repeat treatment. The other patient showed persistent biopsy-proven AMR, and dd-cfDNA levels remained >1% despite ongoing treatment.
*Conclusions: Consistent with previous studies, all pediatric renal transplant recipients with dd-cfDNA >1% had BPAR that correlated with de novo DSA development. Patients treated for BPAR demonstrated a significant dd-cfDNA decrease associated with stable CrCl. Persistently elevated dd-cfDNA levels suggest ongoing graft injury, indicating the need for re-evaluation and biopsy. Ongoing investigation is warranted to support dd-cfDNA utility in the management of acute rejection for pediatric renal transplant recipients.
To cite this abstract in AMA style:
Klein KA, Kincaide EL, Fei M, Bell AM, Arar MY, Ranch D. Surveillance of Donor-Derived Cell-Free DNA Throughout Treatment of Acute Rejection in Pediatric Renal Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/surveillance-of-donor-derived-cell-free-dna-throughout-treatment-of-acute-rejection-in-pediatric-renal-transplant-recipients/. Accessed February 22, 2026.« Back to 2022 American Transplant Congress