Superior Renal Function Sustained for 24 Months through Early Everolimus-Facilitated Reduction of Tacrolimus Versus Standard Tacrolimus in De Novo Liver Transplant Recipients: Results of a Randomized Trial
For the H2304 Study Group, Pisa, Italy
Novartis Pharma AG, Basel, Switzerland
Novartis Pharmaceuticals Corporation, New Jersey
Meeting: 2013 American Transplant Congress
Abstract number: 450
mTOR inhibitors have the potential to reduce calcineurin inhibitor nephrotoxicity by minimizing or eliminating the need for their use. The 12 month (M) results of H2304 (NCT00622869) study demonstrated superior renal function with everolimus (EVR) plus reduced tacrolimus (rTAC) vs. standard TAC (TAC-C) in de novo liver transplant recipients (LTxR). Presented here are 24M renal function results.
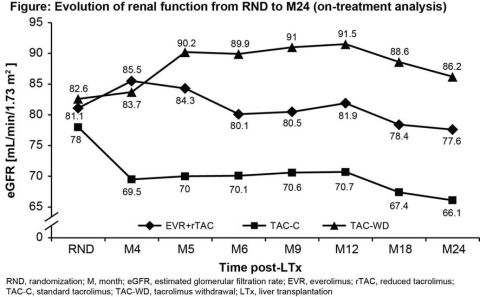
For this 24M, multicenter, open-label study 719 de novo LTxR were randomized (1:1:1) after a 30-day (±5 days) run-in period with TAC (±mycophenolate mofetil), to receive either EVR (C0 3-8 ng/mL) with rTAC (C0 3-5 ng/mL; EVR+rTAC, N=245) or EVR (C0 6-10 ng/mL) with TAC withdrawal (TAC-WD; N=231) at M4 or TAC-C (C0 6-10 ng/mL; TAC-C, N=243); all patients received corticosteroids. Enrollment in TAC-WD arm was stopped early due to higher rejection rates. Main endpoints at M24 included composite efficacy failure rate of treated biopsy proven acute rejection, graft loss or death, and evolution of renal function from randomization (RND) to M24 measured as eGFR by MDRD4.
At M24, composite efficacy failure rate in EVR+rTAC arm was comparable to TAC-C (10.3% vs. 12.5%, p=0.452). Evolution of renal function from RND to M24 was superior for EVR+rTAC vs. TAC-C with an adjusted mean difference in eGFR change of 6.66 mL/min/1.73m2 (p=0.0018; ITT population). Significantly higher eGFR with EVR+rTAC was achieved at M2 post-LTx and was maintained until M24. On-treatment data showed a decrease in mean eGFR from RND to M24 of 6.6 mL/min/1.73m2 with EVR+rTAC vs. 13 mL/min/1.73m2 with TAC-C and 2.5 mL/min/1.73m2 gain with TAC-WD. Urinary protein:creatinine ratio (mg/g) at M24 was higher with EVR+rTAC vs. TAC-C (Mean±SD: 194±280 vs. 159±284, p=0.006).
Early introduction of EVR at 1M post-LTx with rTAC showed superior renal function sustained for 24M compared to TAC-C, without compromising efficacy in de novo LTxR.
De Simone, P.: Grant/Research Support, Novartis. Kintmalm, G.: Grant/Research Support, Novartis, Baylor, Pfizer, Quark, Astellas, OPO. McCormick, P.: Grant/Research Support, Novartis, Astellas, Roche, MSD, Bayer. Lopez, P.: Employee, Novartis. Junge, G.: Employee, Novartis. Dong, G.: Employee, Novartis. Joseph, D.: Employee, Novartis. Duvoux, C.: Grant/Research Support, Novartis, Astellas, Roche, Other, Astellas, Speaker's Honoraria and Travel Grants.
To cite this abstract in AMA style:
Simone PDe, Detry O, Kintmalm G, Goss J, McCormick P, Rossi M, Moya A, Lopez P, Junge G, Dong G, Joseph D, Duvoux C. Superior Renal Function Sustained for 24 Months through Early Everolimus-Facilitated Reduction of Tacrolimus Versus Standard Tacrolimus in De Novo Liver Transplant Recipients: Results of a Randomized Trial [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/superior-renal-function-sustained-for-24-months-through-early-everolimus-facilitated-reduction-of-tacrolimus-versus-standard-tacrolimus-in-de-novo-liver-transplant-recipients-results-of-a-randomized/. Accessed March 9, 2026.« Back to 2013 American Transplant Congress
