Stability of T Reg with Combination of mTOR Inhibitors with Belatacept in Reducing Risk of Acute Rejection in Kidney Transplant
University of California San Francisco (UCSF), San Francisco, CA
Meeting: 2020 American Transplant Congress
Abstract number: 227
Keywords: Co-stimulation, Immunosuppression, Kidney transplantation, Rejection
Session Information
Session Name: Novel Tools to Assess Immunosuppressive Efficacy
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:03pm-4:15pm
Presentation Time: 4:03pm-4:15pm
Location: Virtual
*Purpose: Belatacept is a costimulatory blocker that is used as de novo maintenance immunosuppression in kidney transplantation to avoid toxic effects of calcineurin inhibitors and improve long term outcomes. In view of experimental studies showing synergy between costimulatory blockade and mTOR inhibitors, we developed a belatacept regimen incorporating everolimus to reduce acute rejection. We performed mechanistic studies to analyze circulatory T cells by flow and functional assays pre-and post-transplant to identify favorable lymphocyte profile that may predict those at lower risk of acute rejection in belatacept-based regimen.
*Methods: We prospectively enrolled 65 kidney transplant recipients (31 deceased; 34 living donors) at our center to receive denovo belatacept from September 2012 to June 2018. PBMCs were collected prior to transplantation, 3 months, and 6 months post-transplant. All patients received thymoglobulin for induction with belatacept 10mg/kg administered on POD 1, 4, 14, 28, 56, and 84. Monthly maintenance dose of 5mg/kg was given starting week 16. Patients were started initially on MPA but were converted to everolimus after 1 month, and all patients were maintained on prednisone.
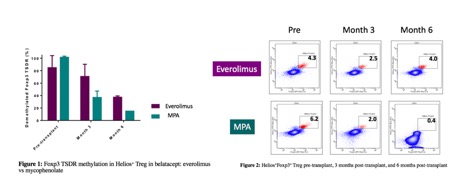
*Results: In the first 12 months post-transplant, 16.9% developed acute rejection: 2 patients were noted to have ACR 1a, 1 with ACR 2a, 6 with ACR 2b, 1 with AMR, and 1 with simultaneous ACR 1a and AMR (at 9 months). All 11 rejections occurred in those who were on MPA and not on mTORi. In addition, 12 patients were found to have borderline rejection on protocol biopsies (7 on mTORi, 5 on MPA). 42 patients did not have any inflammation on biopsies. Patients who had biopsy-proven rejection or borderline changes had significantly higher percentages of CD8+CD28–T cells, and those who had rejection with low CD8+CD28- were found to have high CD2hiCD28hiin CD8+CD45RO+ T cells. Helios+Foxp3+ Treg population was higher at 3 months and 6 months post-transplant in everolimus group compared to mycophenolate group.
*Conclusions: Acute rejection in belatacept-based regimen can be reduced by combining belatacept with mTORi due to it’s synergistic effect on memory CD8+CD28-CD38+ cells that are refractory to costimulatory blockade. Pretransplant immunotyping of circulating T cells to identify those with low percentage of CD8+CD28- & CD2hiCD28hi in CD8+CD45RO+ may identify patients who will be at lowest risk of rejection on belatacept. The stability of T regs in everolimus-based regimen post-transplant also may contribute to reducing acute rejection.
To cite this abstract in AMA style:
Shoji J, Leung J, Tang Q, Vincenti F. Stability of T Reg with Combination of mTOR Inhibitors with Belatacept in Reducing Risk of Acute Rejection in Kidney Transplant [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/stability-of-t-reg-with-combination-of-mtor-inhibitors-with-belatacept-in-reducing-risk-of-acute-rejection-in-kidney-transplant/. Accessed March 9, 2026.« Back to 2020 American Transplant Congress