Single-Dose Pharmacokinetics and Pharmacodynamics of Belatacept in Adolescent Kidney Transplant Recipients
1Children's National Medical Center, Washington, DC
2Washington University, St. Louis, MO
3University of Alabama, Birmingham, AL
4Emory University & Children's Healthcare of Atlanta, Atlanta, GA
5Bristol-Myers Squibb, Lawrenceville, NJ
6University of California, Los Angeles, CA.
Meeting: 2018 American Transplant Congress
Abstract number: B212
Keywords: Immunosuppression, Kidney transplantation, Pediatric, Pharmacokinetics
Session Information
Session Name: Poster Session B: Kidney: Pediatrics
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Purpose: To assess the pharmacokinetics (PK) and pharmacodynamic (PD) effect of a single dose of belatacept (bela) administered to adolescent kidney transplant recipients (KTR).
Methods: In a phase I trial, 9 EBV-positive KTR aged 13–17 yrs (mean 15.1 yrs) on calcineurin inhibitor-based immunosuppression for >6 months post-transplant received one IV bela infusion (7.5 mg/kg, 30 min). Blood was collected for PK serially over Days 1–57. PD was measured by % CD86 receptor occupancy (%CD86RO). Blood was collected for %CD86RO on Days 1, 29, and 57. Adverse events (AEs) were recorded to Day 57 and serious AEs (SAEs) to Month 6.
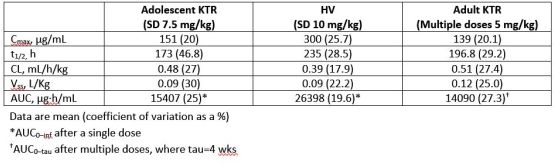
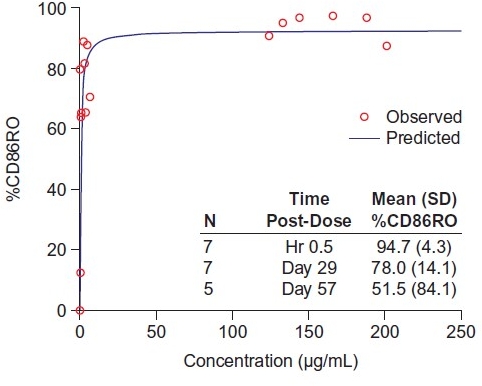
Results: All 9 completed the study. Mean half-life (t1/2), volume of distribution (Vss), and systemic clearance (CL) in adolescent KTR were comparable to historical values from healthy adult volunteers (HV) and adult KTR (Table). Mean %CD86RO increased with increasing bela concentration, suggesting a pharmacologic PK/PD relationship (Figure). Four SAEs were reported; none was deemed related to bela.
Conclusions: In adolescent KTR, the range of PK values was similar to those seen in HV and adult KTR. The PK/PD relationship between %CD86RO and bela concentration was similar to that seen in adults, providing a basis for dose selection in future adolescent studies. A single dose of bela was well tolerated in the 9 adolescent KTR. Presented at ASN Kidney Week 2017 in New Orleans, LA.
CITATION INFORMATION: Moudgil A., Dharnidharka V., Feig D., Warshaw B., Perera V., Murthy B., Roberts M., Polinsky M., Ettenger R. Single-Dose Pharmacokinetics and Pharmacodynamics of Belatacept in Adolescent Kidney Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Moudgil A, Dharnidharka V, Feig D, Warshaw B, Perera V, Murthy B, Roberts M, Polinsky M, Ettenger R. Single-Dose Pharmacokinetics and Pharmacodynamics of Belatacept in Adolescent Kidney Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/single-dose-pharmacokinetics-and-pharmacodynamics-of-belatacept-in-adolescent-kidney-transplant-recipients/. Accessed March 9, 2026.« Back to 2018 American Transplant Congress