Risk of Infection in Treatment of Acute Cellular Rejection in Heart Transplant Recipients
Tampa General Hospital, Tampa, FL
University of North Carolina, Chapel Hill, NC.
Meeting: 2018 American Transplant Congress
Abstract number: B63
Keywords: Heart transplant patients
Session Information
Session Name: Poster Session B: Heart and VADs: All Topics
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
There has been no publication of the incidence of infection (infxn) after the treatment of acute cellular rejection (ACR) in heart transplant recipients (HTR). We sought to determine the incidence of infxn after treatment of ACR with anti-thymocyte globulin rabbit (rATG) and high dose corticosteroids (hCS) and secondarily to compare time to first infxn and risk factors associated with infxn.
An analysis was conducted at a single center from 10/2011-10/2016 and included all HTR with at least one episode of treated ACR. Multi-organ recipients were excluded. Risk factors and 6 month incidence of any infxn following ACR was assessed. Bacterial infxn was defined as any positive culture treated for a minimum of 72 hours. Viral infxn included any positive PCR, Ag test, or CMV PCR > 200 copies/mL. Fungal infxn was defined as a positive culture, excluding candidiasis or yeast in urine or sputum (<104 cfu/mL).
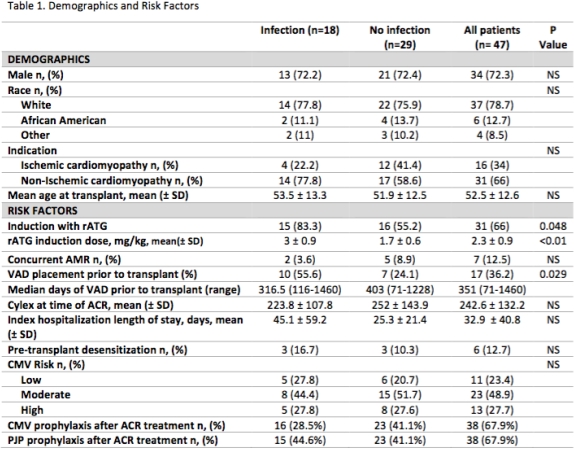
Of 227 HTRs at this center, 47(21%) patients were treated with hCS and/or rATG for a total of 56 episodes of ACR. Eighteen patients (38%) developed infxn with 20 episodes of ACR; 5 were treated with rATG and 15 hCS alone. Median time to first episode of ACR was 57 days from date of transplant and median time to infxn was 67 days from ACR treatment. Incidence of bacterial, fungal, and viral infxns were 75%, 21%, and 17% respectively. More patients received rATG induction at time of transplant in the infxn vs no infxn group (83% vs 55%, p=0.048) with higher cumulative rATG mg/kg doses (2.9±0.9 vs 1.7±0.6). VAD placement before transplant was significantly higher in the infxn cohort (55% vs 24%, p=0.03). Race, gender, age, indication, pre-transplant desensitization, hospital length of stay, and CMV risk were not found to be significant risk factors for infxn. Most patients were receiving CMV (69%) and PJP prophylaxis (67%) at time of ACR as continuation of the post-transplant protocol (Table 1).
Infxn following treatment of ACR is common with bacterial infxns as the most prevalent. Risk factors for infxn following treatment of ACR were non-modifiable and include rATG for induction and VAD prior to transplant.
CITATION INFORMATION: Fallah T., Logan A., Rumore A., Doligalski C. Risk of Infection in Treatment of Acute Cellular Rejection in Heart Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Fallah T, Logan A, Rumore A, Doligalski C. Risk of Infection in Treatment of Acute Cellular Rejection in Heart Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/risk-of-infection-in-treatment-of-acute-cellular-rejection-in-heart-transplant-recipients/. Accessed March 6, 2026.« Back to 2018 American Transplant Congress