Renal Transplants In Hepatitis C Negative Recipients With Nucleic Acid Positive Donors Using Two Weeks Of DAA Prophylaxis (Rehanna – 2)
N. M. Desai1, S. Leung1, J. Motter1, F. Naqvi2, N. Bair1, B. Barnaba2, D. Warren1, D. Segev1, C. Durand2
1Surgery, Johns Hopkins University, Baltimore, MD, 2Medicine, Johns Hopkins University, Baltimore, MD
Meeting: 2022 American Transplant Congress
Abstract number: 9021
Keywords: Hepatitis C, Kidney transplantation
Topic: Clinical Science » Infection Disease » 27 - Non-Organ Specific: Viral Hepatitis
Session Information
Session Name: Late Breaking: Clinical
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 4:50pm-5:00pm
Presentation Time: 4:50pm-5:00pm
Location: Hynes Room 313
*Purpose: To mitigate the organ shortage and underutilization of hepatitis C-infected deceased donor (HCV D+) organs, we evaluated the use of HCV D+ kidneys in HCV-uninfected recipients (HCV R-) with a short-course (2 weeks) of direct-acting antiviral (DAA) prophylaxis in a pilot trial (NCT04575896).
*Methods: HCV-negative kidney transplant candidates ≥ 18 years old were eligible. HCV D+ were ≤ 60 years old with a reactive HCV nucleic acid test, and terminal creatinine ≤ 5.0 mg/dL. Donor HCV genotype was determined post-transplant. DAA prophylaxis included one dose of glecaprevir/pibrenstavir pre-transplant and daily dosing for 2 weeks post-transplant. Recipient HCV RNA was measured post-transplant on treatment days 1, 4, 7, and week 2. Recipient HCV RNA was also measured post treatment at weeks 1, 2, 4, and 12.
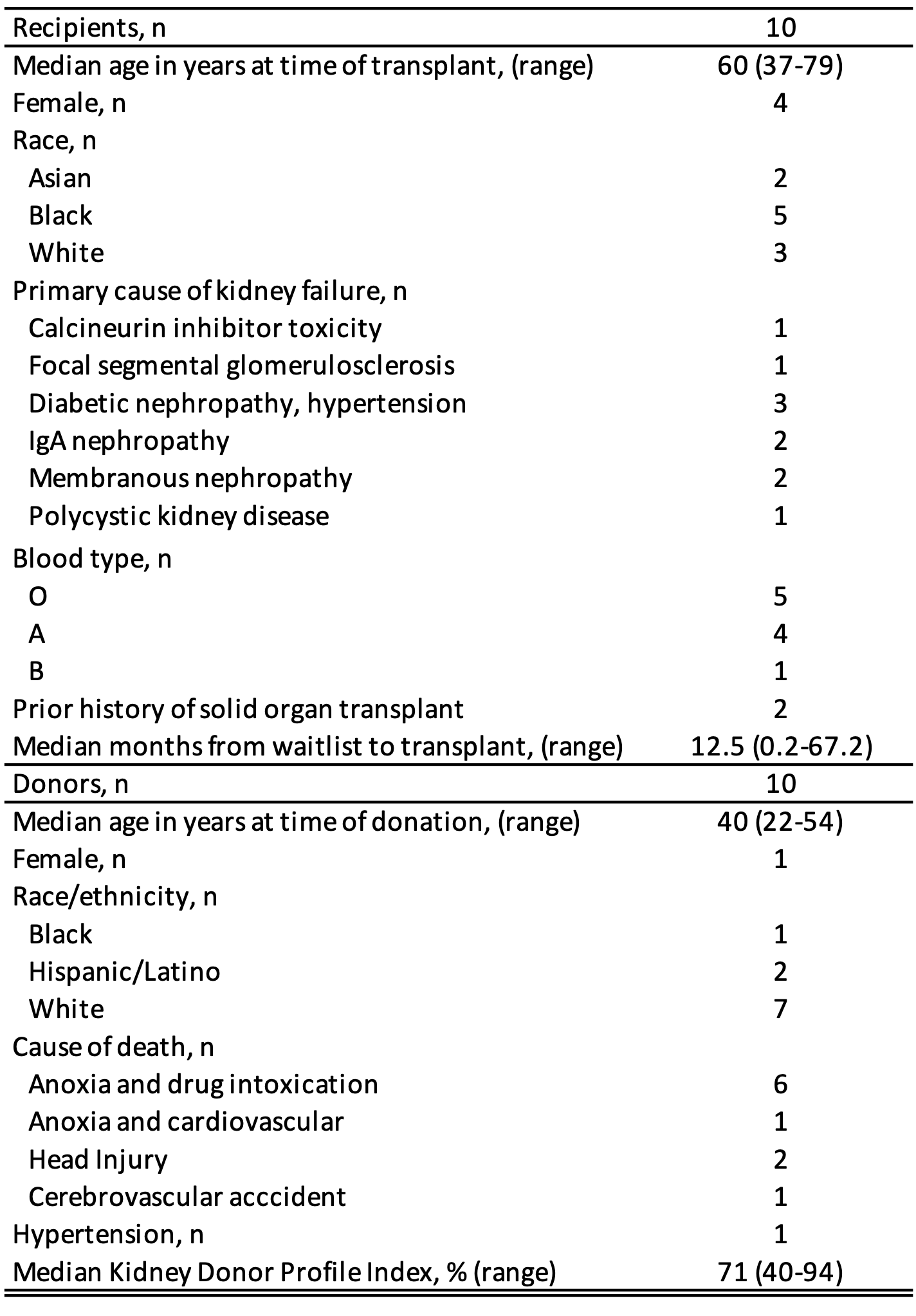
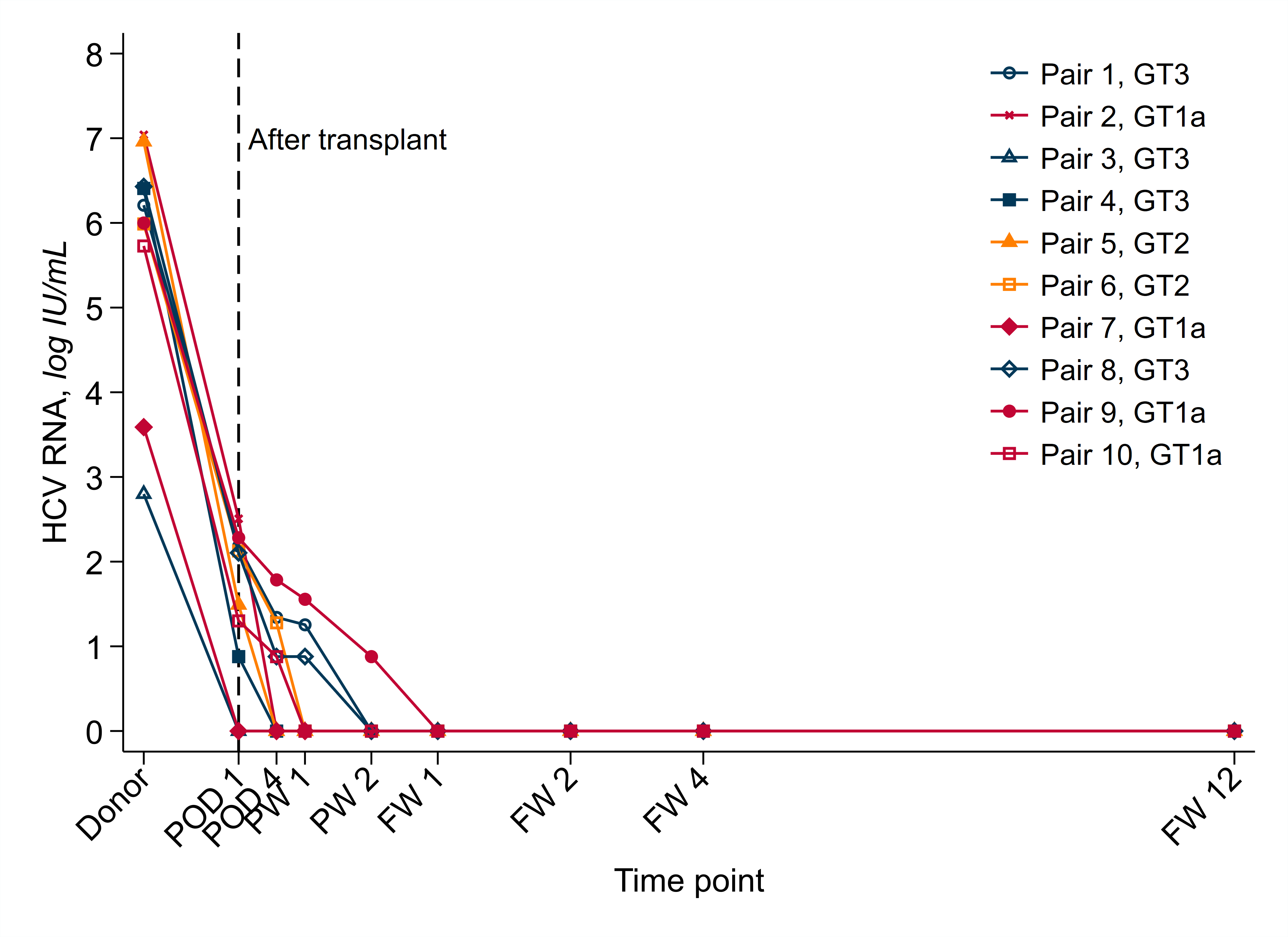
*Results: Since 11/2020, we have performed 10 HCV D+/R- kidney transplants. Median donor age was 40, median KDPI 71; drug overdose was the cause of donor death in most (Table 1). Donor HCV RNA range was 629 to 1.1 X 107 IU/mL (median 1.31 X 106) with genotypes 1a (n=4), 2 (n=2), and 3 (n=4). One recipient had a grade 3 hyperbilirubinema possibly related to DAAs. Three recipients had no quantifiable HCV viremia post-transplant; seven had quantifiable low-level HCV viremia in the early posttransplant period. All ten recipients completed 2 weeks of DAAs and all ten recipients had an undetectable HCV RNA 12 weeks following completion of treatment. There was one graft failure 4.5 months post-transplant related to interstitial scarring.
*Conclusions: This pilot study provides proof-of-concept that 2-week DAA prophylaxis in HCV D+/R- kidney transplantation is well-tolerated and prevents transmission of HCV to the recipient.
To cite this abstract in AMA style:
Desai NM, Leung S, Motter J, Naqvi F, Bair N, Barnaba B, Warren D, Segev D, Durand C. Renal Transplants In Hepatitis C Negative Recipients With Nucleic Acid Positive Donors Using Two Weeks Of DAA Prophylaxis (Rehanna – 2) [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/renal-transplants-in-hepatitis-c-negative-recipients-with-nucleic-acid-positive-donors-using-two-weeks-of-daa-prophylaxis-rehanna-2/. Accessed February 22, 2026.« Back to 2022 American Transplant Congress