Reduction in Opioid Discharge Prescribing for Kidney Transplant Recipients Through Implementation of a Standardized Opioid Prescribing Policy
1Pharmacy, University of Rochester Medical Center, Rochester, NY, 2Transplant Surgery, University of Rochester Medical Center, Rochester, NY, 3Wegmans School of Pharmacy, St. John Fisher College, Rochester, NY
Meeting: 2022 American Transplant Congress
Abstract number: 597
Keywords: Kidney, Outcome, Pain, Post-operative complications
Topic: Administrative » Administrative » 01 - Quality Assurance Process Improvement & Regulatory Issues
Session Information
Session Name: Quality Assurance Process Improvement & Regulatory Issues
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:50pm-7:00pm
Presentation Time: 6:50pm-7:00pm
Location: Hynes Room 206
*Purpose: Diversion of unused opioid medications has contributed to the opioid epidemic, and opioid use after kidney transplantation has been shown to be a risk factor for mortality, graft loss, and medication non-adherence. The purpose of this study was to evaluate the effectiveness of an opioid prescribing discharge policy after kidney transplantation.
*Methods: A retrospective single-center analysis of a quality improvement project to reduce opioid prescribing after kidney transplantation in opiate non-tolerant subjects was conducted. The prescribing policy incorporated inpatient use of opioids 48 hours prior to discharge to standardize the number of opioid tablets prescribed. Comparisons were made between the intervention group (IntG) and a historical group (HistG) between 8/1/2020 and 7/31/2021.
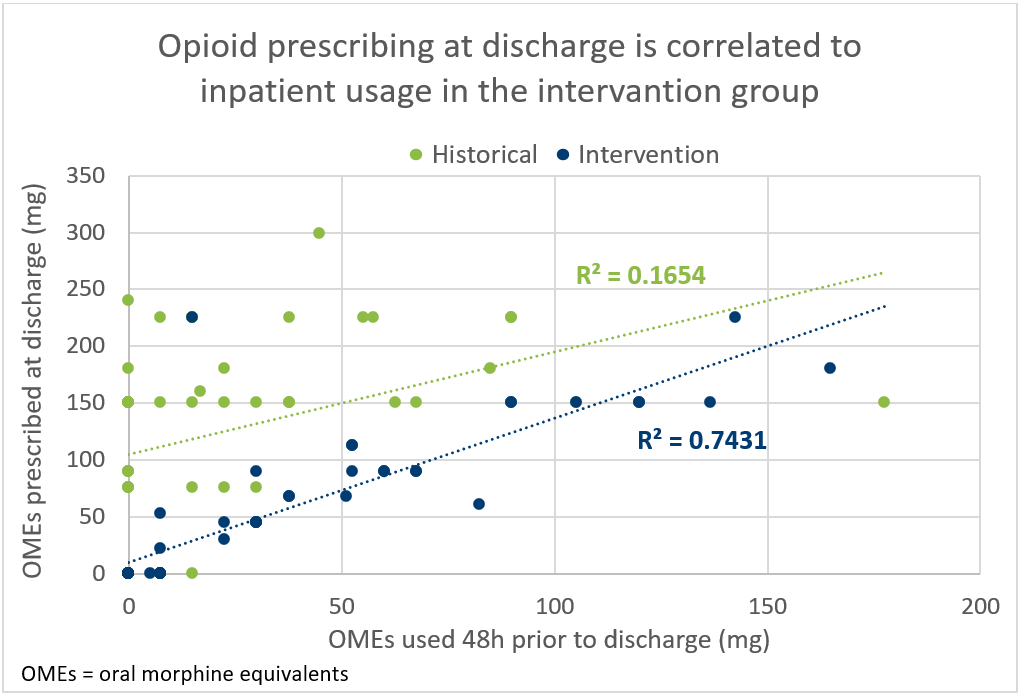
*Results: Discharge opioid prescribing was evaluated for 102 kidney transplant recipients (47 HistG, 55 IntG). Patients were a median of 56 years old (IQR: 44-64 years), 71% were Caucasian, 58% were male, 66% were deceased donor recipients, and 8% had a history of chronic pain. Fewer patients in the IntG received opioids at discharge than the HistG (HistG 81% vs. IntG 53%; p=0.003). Median total oral morphine equivalents (OME) prescribed was reduced (HistG: 150 mg (IQR 150-214 mg) vs. IntG: 90 mg (IQR 53-150 mg); p <0.001). No patient requested additional opioids before their first clinic visit in either group. Opioids prescribed after discharge in the first 30 days were not different (p=0.659). Two factors, being in the IntG (OR 0.18, 95% CI 0.07-0.48) and older age (OR 0.95, 95% CI 0.91-0.99), were independent predictors of being discharged without an opioid prescription. After policy implementation, opioid prescribing at discharge was more closely correlated to inpatient usage (correlation coefficient 0.1654 HistG vs. 0.7431 IntG) (Figure 1).
*Conclusions: A standardized protocol for opioid prescribing at discharge after kidney transplantation significantly reduced the percentage of patients receiving an opioid as well as the quantity dispensed.
To cite this abstract in AMA style:
Fredrick S, Dokus K, Cooley J, Dulen A, Melaragno J. Reduction in Opioid Discharge Prescribing for Kidney Transplant Recipients Through Implementation of a Standardized Opioid Prescribing Policy [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/reduction-in-opioid-discharge-prescribing-for-kidney-transplant-recipients-through-implementation-of-a-standardized-opioid-prescribing-policy/. Accessed March 9, 2026.« Back to 2022 American Transplant Congress