Reducing HIV / HCV Infection Transmission from Organ Transplantation through Nucleic Acid Testing of All Potential Deceased Donors: A Cost-Effectiveness Analysis
UCSF, San Francisco
Meeting: 2013 American Transplant Congress
Abstract number: 480
Background: Compared to antibody (Ab) tests, nucleic acid-amplification tests (NAT) shorten the window period during which HIV/HCV infection cannot be detected. We aimed to evaluate the cost-effectiveness of HIV/HCV NAT for all donors from the societal perspective.
Methods: We created a decision model to estimate the cost-utility of HIV and HCV NAT vs. HIV and HCV Ab tests alone. The primary outcome was the incremental cost effectiveness ratio (ICER) = net costs of NAT testing per quality-adjusted life year (QALY) gained, for a range of HIV/HCV prevalences and NAT costs. Estimates of infection risk, lifetime costs of infection, and QALYs gained were obtained through a comprehensive literature search. Key input values included: 8 and 5 QALYs gained for each HIV and HCV infection averted, respectively; 3.6 transplants per donor; and lifetime costs of $385,200 for HIV and $54,596 for HCV infection.
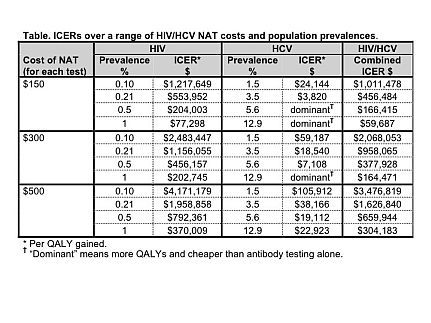
Results: The Table shows the ICER over a range of NAT costs and HIV/HCV prevalences. For HIV, the ICER ranged from $77,298 to $4,171,179 per QALY gained. For HCV, the ICER ranged from dominant (more QALYs and saves money) to $105,912 per QALY gained. The ICER for combined HIV/HCV NAT ranged from $59,687 to $3,476,819. After including the lost value of transplantation due to a 0.05% false positive rate for NAT, the cost per QALY gained for HIV NAT rises from $0.5 million for the high prevalence/low cost scenario to $8.8 million for the low prevalence/high cost scenario.
Conclusions: Given the higher prevalence of HCV and improved window detection period of HCV NAT vs. HCV Ab testing, universal HCV NAT for all donors appears cost effective to reduce infection transmission in solid organ transplantation. Conversely, HIV NAT appears cost ineffective except for scenarios with very high prevalence and low costs. Based on this model, we recommend universal HCV NAT for all solid organ transplant donors, but implementation of HIV NAT should depend on the HIV prevalence within a population and the cost of HIV NAT per donor.
To cite this abstract in AMA style:
Lai J, Kahn J, Tavakol M, Peters M, Roberts J. Reducing HIV / HCV Infection Transmission from Organ Transplantation through Nucleic Acid Testing of All Potential Deceased Donors: A Cost-Effectiveness Analysis [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/reducing-hiv-hcv-infection-transmission-from-organ-transplantation-through-nucleic-acid-testing-of-all-potential-deceased-donors-a-cost-effectiveness-analysis/. Accessed March 3, 2026.« Back to 2013 American Transplant Congress
