Prospective Randomized Controlled Study Evaluating the Relationship Between Electronic Adherence Data and Tacrolimus Concentration Data.
1University of Chicago Transplant Center, University of Chicago, Chicago, IL
2Clinical Research, Innovation Clinical Research, LLC, Tucson, AZ
3R&D, SIMpill, LLC, Madison, WI.
Meeting: 2016 American Transplant Congress
Abstract number: C73
Keywords: FK506, Immunosuppression
Session Information
Session Name: Poster Session C: Economics, Public Policy, Allocation, Ethics
Session Type: Poster Session
Date: Monday, June 13, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Purpose: The SIMpill® Medication Adherence System (MAS) is being evaluated in transplant recipients in a prospective 3-year study. The MAS captures patient medication adherence data in real-time. This report describes preliminary results combining adherence data and tacrolimus levels.
Methods: 89 transplant recipients were randomized to 1 of 4 groups: 2 intervention groups (I1 and I2) and 2 control groups (C1 and C2). Subjects in I1 or I2 received a MAS, a medication-dispensing device that communicates timing of openings or doses taken to a secure server. If a dose is missed, a text message reminder is sent to the subject (I1). In addition to patient reminders, providers were notified if consecutive doses were missed over a 72 hour period (I2), enabling intervention. C1 received a MAS but feedback was not provided to the patient or provider. C2 did not receive a MAS device. The MAS utilizes patient dose timing data with a one-compartment pharmacokinetic (PK) model to project individual tacrolimus drug level plots. The individual tacrolimus levels were plotted on drug curves based on the adherence data.
Results: PK modeling utilizing MAS data combined with tacrolimus levels resulted in 4 adherence profiles: A) patients with consistent dose timing with a blood draw time close to patient's planned dose time, B) patients with irregular dose timing with a blood draw time close to patient's planned dose time, C) patients with consistent dose timing with a blood draw after the dose time resulting in a low trough level, and D) patients who do not hold their dose before the blood draw, resulting in the perception of a high trough level 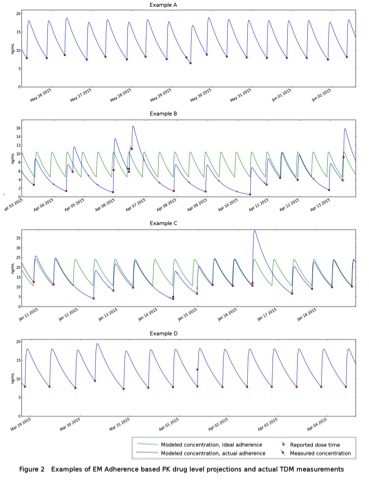 .
.
Conclusions: Using a MAS system may improve the interpretation of actual serum tacrolimus levels. Preliminary results indicate that the MAS may provide useful PK projections of tacrolimus levels. Irregular dose administration results in variable and/or inaccurate tacrolimus levels.
CITATION INFORMATION: Kane B, Lockwood M, Potter L, Lourenco L, Bodzin A, Covington D, Mayer K, Millis J. Prospective Randomized Controlled Study Evaluating the Relationship Between Electronic Adherence Data and Tacrolimus Concentration Data. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Kane B, Lockwood M, Potter L, Lourenco L, Bodzin A, Covington D, Mayer K, Millis J. Prospective Randomized Controlled Study Evaluating the Relationship Between Electronic Adherence Data and Tacrolimus Concentration Data. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/prospective-randomized-controlled-study-evaluating-the-relationship-between-electronic-adherence-data-and-tacrolimus-concentration-data/. Accessed March 9, 2026.« Back to 2016 American Transplant Congress
