Preliminary Results from a Multicenter National Trial of Antiviral-Facilitated Transplantation of Hepatitis C Hearts to Expand the Donor Pool
Baylor University Medical Center, Dallas, TX
Meeting: 2019 American Transplant Congress
Abstract number: 298
Keywords: Hepatitis C
Session Information
Session Name: Concurrent Session: Donor and Recipient Selection in Heart Transplanation
Session Type: Concurrent Session
Date: Monday, June 3, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:06pm-3:18pm
Presentation Time: 3:06pm-3:18pm
Location: Room 206
*Purpose: Solid organs from donors with evidence of active HCV viremia are historically underutilized particularly for HCV negative recipients. The development of easily tolerated, oral, direct-acting antiviral agents has begun changing that landscape. Safety data is emerging from patients transplanted under research protocols and under best-interest standards.
*Methods: Our center has transplanted hearts from donors with active HCV viremia into HCV-negative recipients both under the best-interest standard and via a national multi-center open-label clinical trial (TROJAN-C, NCT03383419). In both settings, our protocols are pre-emptive rather than universal prophylaxis, initiating therapy only after demonstrable transmission of viremia. Patients enrolled in the trial receive a 12-week course of the pangenotypic sofosbuvir/velpatasvir (SOF/VEL) initiated within 14 days of quantifiable viremia, whereas best-interest patients initiate therapy during a wider time window.
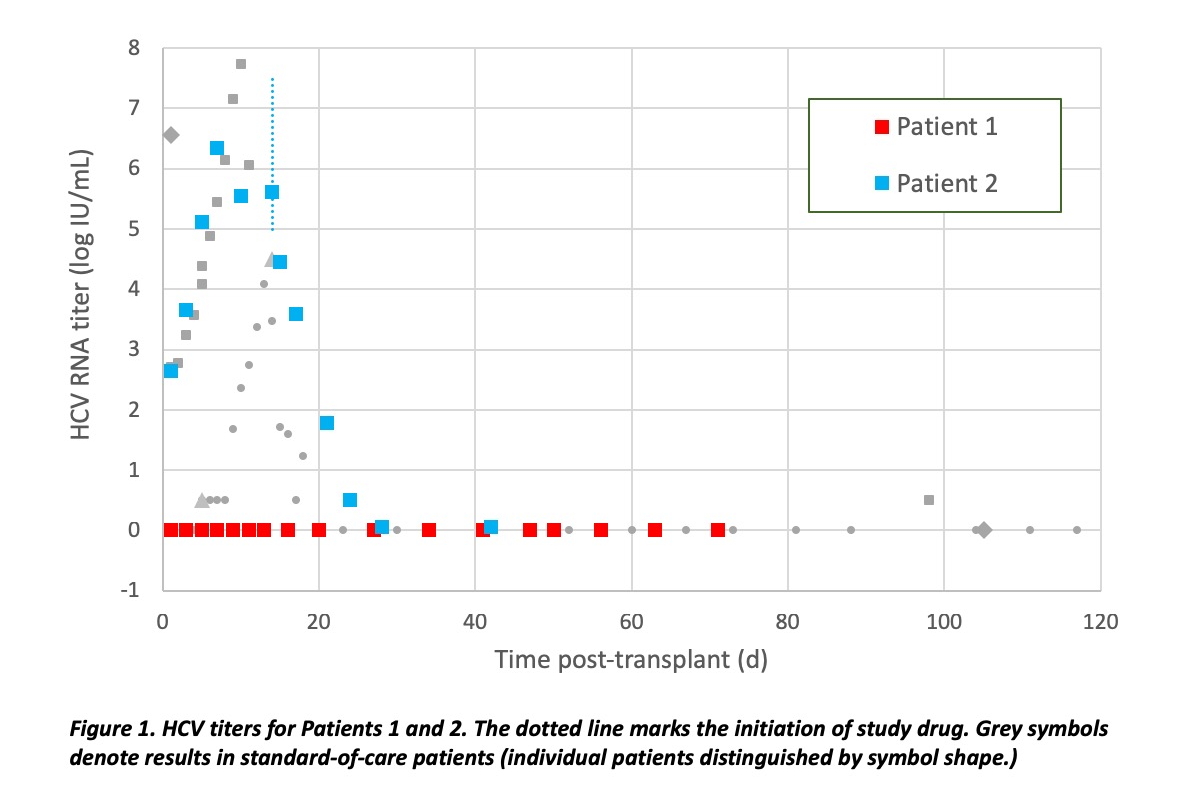
*Results: A minority of clinical trial patients, consented for the first available donor heart independent of HCV viremia, have received transplants from HCV viremic donors. Patient 1 in TROJAN-C received a heart from an HCV nucleic acid test (NAT)-positive donor. At the time of harvest, a sample for quantitative HCV PCR was obtained that was also positive and quantifiable, albeit with a titer too low to allow successful genotyping. 3 months post-transplant, HCV remained undetectable, diagnostic for non-transmission. Patient 2 followed a course similar to other patients at our center who received a heart transplant from HCV-positive donor. All donor-derived HCV at our center have either achieved SVR-12 or are in process to achieve SVR-12 (or non-transmission).
*Conclusions: Cardiac donors with HCV viremia are being utilized in multiple centers, creating competition for this scarce resource. HCV-positive donors are not a panacea for the donor shortage. Although patients are counseled to expect transmission of HCV from an HCV-viremic donor, the infectivity rate has been <100%. In contrast to other centers reporting incomplete infectivity, the trial has provided the opportunity to confirm HCV viremia both by nucleic-acid testing and quantitative PCR, provided additional data against false-positive assessment of viremia. Evidence of occasional non-transmission supports the safety and efficacy of pre-emptive antiviral treatment rather than universal prophylaxis against HCV post-transplant for prevention of resistance and cost-effectiveness.
To cite this abstract in AMA style:
Martits-Chalangari K, Doss AK, Clark M, Felius J, Hall SA, Gottlieb RL. Preliminary Results from a Multicenter National Trial of Antiviral-Facilitated Transplantation of Hepatitis C Hearts to Expand the Donor Pool [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/preliminary-results-from-a-multicenter-national-trial-of-antiviral-facilitated-transplantation-of-hepatitis-c-hearts-to-expand-the-donor-pool/. Accessed March 7, 2026.« Back to 2019 American Transplant Congress