Precision Medicine for Determining the Efficacy of a Novel Belatacept Regimen
Transplant Nephrology, UCSF, San Francisco, CA.
Meeting: 2018 American Transplant Congress
Abstract number: 123
Keywords: Co-stimulation, Immunosuppression, Kidney transplantation, Rejection
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: Co-Stimulation Based Regimens
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:42pm-5:54pm
Presentation Time: 5:42pm-5:54pm
Location: Room 6C
Intro
Belatacept is increasingly used as maintenance immunosuppression to improve long term outcomes in kidney transplant. Since acute rejection in patients on belatacept have been noted to be more frequent and histologically severe, identifying patients with Precision Medicine who will benefit from belatacept is important. We investigated pretransplant recipient immune profiles to determine which lymphocyte population would be best predictor in identifying those who will be at lowest risk for costimulation blockade-resistant rejection.
Methods
We prospectively enrolled 20 kidney transplant recipients (8 DDRT; 12 living) at our center to receive denovo belatacept from May 2016 to March 2017. PMBCs were collected prior to transplant and at the time of biopsies. All patients received thymoglobulin 3mg/kg for induction and were maintained on belatacept. Patients were initially on MMF but were converted to mTORi after 1 month and were maintained on prednisone 5mg daily. Protocol biopsies were performed at 6 months.
Results
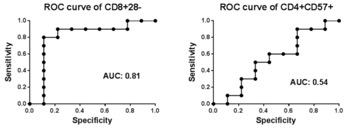
On cause biopsies, 2 patients had ACR 1a (at 4 wks; 6 wks), 1 with ACR 2b (at 2 mo), and 1 with AMR (at 4 mo). 6 patients had borderline rejection on protocol biopsy. 9 patients did not have any inflammation. 3 of 4 rejections occurred in those who were on MMF. 18 remained on belatacept and 2 were converted to tacrolimus. No correlation was found between rejection and CD4+CD57+T cell % in pretransplant PBMC. Patients with rejection or borderline changes had significantly higher % of CD8+CD28-T cells in pretransplant PBMC compared to those who had normal biopsies. Those with greater than 50% of CD8+CD28– T cells pretransplant were more likely to experience rejection (OR 18.7, p=0.02). Rejection was associated with elevated ex-vivo donor antigen-stimulated TNFα production by CD8+CD28– T cells.
Conclusion
CD28-CD8+T cells are associated with belatacept-resistant rejection. Pretransplant immunotyping may provide useful tool for identifying patients suitable for belatacept therapy.
| at 6 mo | Total (N=20) | Rejection (N=4) |
| Serum creat (mg/dL) | 1.06 | 1.04 |
| eGFR (mL/min) | 70 | 65 |
| U prot/creat (g/g) | 0.22 | 0.21 |
CITATION INFORMATION: Shoji J., Leung J., Tavares E., Tang Q., Vincenti F. Precision Medicine for Determining the Efficacy of a Novel Belatacept Regimen Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Shoji J, Leung J, Tavares E, Tang Q, Vincenti F. Precision Medicine for Determining the Efficacy of a Novel Belatacept Regimen [abstract]. https://atcmeetingabstracts.com/abstract/precision-medicine-for-determining-the-efficacy-of-a-novel-belatacept-regimen/. Accessed February 18, 2026.« Back to 2018 American Transplant Congress