Pharmakokinetics of Daclatasvir, Sofosbuvir and GS-331007 in a Prospective Cohort of HCV Positive Kidney Transplant Recipients.
E. Schrezenmeier,1 P. Galander,1 F. Hoffmann,2 C. Jaeger,2 J. Lisec,2 J. Schrezenmeier,2 O. Staeck,1 L. Lehner,1 D. Khadzhynov,1 F. Halleck,1 M. Duerr,1 K. Budde.1
1Nephrology, Charite, Berlin, Deutschland, Germany
2Molekulares Krebsforschungszentrum, Charite, Berlin, Deutschland (DEU), Germany
Meeting: 2017 American Transplant Congress
Abstract number: A293
Keywords: Hepatitis C, Pharmacokinetics
Session Information
Session Name: Poster Session A: Viral Conundrums
Session Type: Poster Session
Date: Saturday, April 29, 2017
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall D1
Background
Limited data exist on the pharmacokinetic (PK) profile of novel direct acting antivirals in kidney transplant recipients (KTR). Here we report the PK of combinated daclatasvir (DAC) and sofosbuvir (SOF) therapy in a controlled prospective study of HCV positive KTR (EudraCT: 2014-004551-32).
Methods
In this study plasma samples of 16 HCV positive KTR receiving DAC/SOF were collected at 4 time points (0h, 1h, 2h and 4h after dosing) at d1, d7, d14 and d21, w8 and w12 after start of treatment. Inclusion criteria were stable graft function and a GFR>30ml/min. DAC, SOF and GS-331007 (SOF07) (inactive metabolite of SOF) plasma concentrations were determined using ultra-performance liquid chromatography quadrupole time of flight mass spectrometry. GFR is always given in ml/min.
Results
At the end of therapy (12 weeks), HCV RNA was detectable in 0% of KTR. All patients showed a rapid virological response with undetectable HCV RNA at a mean of 21d after start of therapy. CNI dose adjustment was required in 4/16 patients. DAC/SOF therapy was well tolerated with no therapy-associated major adverse events and no drug discontinuations. The mean GFR in our cohort was 56.4 (±15.8). 7/16 patients had a GFR≤60 at baseline. Mean SOF07 trough level were 339.5ng/ml (±174.9) in patients with a GFR≥60 and 404.3ng/ml (±226) in patients with a GFR≤60 at d7. At d84 trough levels were 357.8ng/ml (±200.8) and 404.2 (±70.2) in patients with a GFR≥60 and in patients with a GFR≤60, respectively. Overall there were no relevant SOF07 trough level changes (Fig.1A-C). A GFR≤60 did not affect AUCs of DAC, SOF and SOF07. AUCs of SOF07 were more stable with less deviations than SOF levels (Fig.1D-F).
Conclusion
The administration of DAC/SOF is safe and highly efficient in KTR. An impaired GFR (30-60) does not lead to a dose accumulation of DAC, SOF and SOF07.Future studies should address the PK of SOF based HCV treatment in KTR with a GFR<30.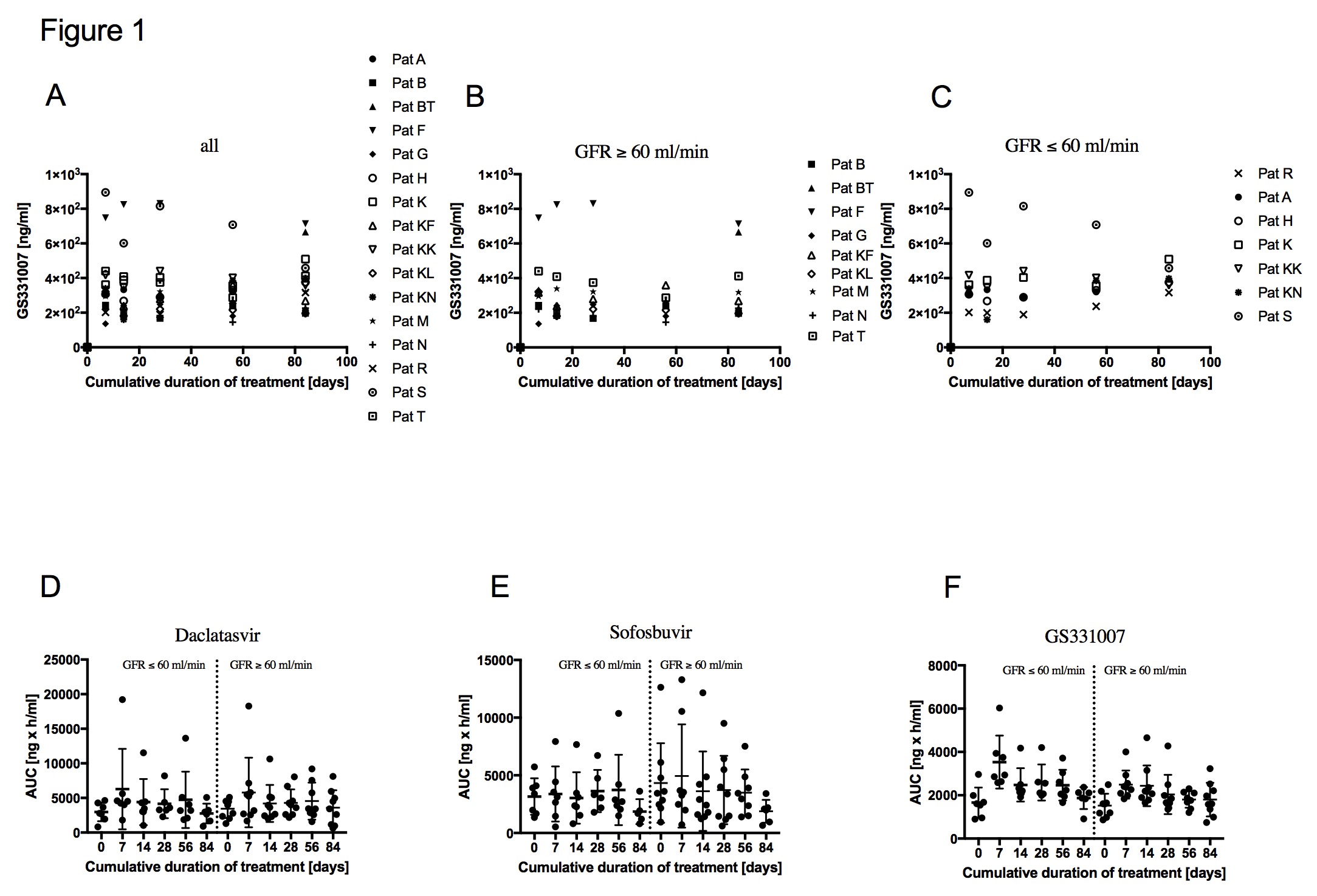
CITATION INFORMATION: Schrezenmeier E, Galander P, Hoffmann F, Jaeger C, Lisec J, Schrezenmeier J, Staeck O, Lehner L, Khadzhynov D, Halleck F, Duerr M, Budde K. Pharmakokinetics of Daclatasvir, Sofosbuvir and GS-331007 in a Prospective Cohort of HCV Positive Kidney Transplant Recipients. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Schrezenmeier E, Galander P, Hoffmann F, Jaeger C, Lisec J, Schrezenmeier J, Staeck O, Lehner L, Khadzhynov D, Halleck F, Duerr M, Budde K. Pharmakokinetics of Daclatasvir, Sofosbuvir and GS-331007 in a Prospective Cohort of HCV Positive Kidney Transplant Recipients. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/pharmakokinetics-of-daclatasvir-sofosbuvir-and-gs-331007-in-a-prospective-cohort-of-hcv-positive-kidney-transplant-recipients/. Accessed February 19, 2026.« Back to 2017 American Transplant Congress
