Pharmacokinetic Evaluation of Astagraf XL® and Prograf® in Renal Transplant Candidates Following Laparoscopic Sleeve Gastrectomy.
1University of Cincinnati, Cincinnati
2Cincinnati Children's Hospital, Cincinnati
3University of Colorado, Denver.
Meeting: 2016 American Transplant Congress
Abstract number: C238
Keywords: FK506, Pharmacokinetics
Session Information
Session Name: Poster Session C: Poster Session 1: Kidney Complications-Other
Session Type: Poster Session
Date: Monday, June 13, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Purpose: The impact of laparoscopic sleeve gastrectomy (LGS) on immunosuppressive drug pharmacokinetics (PK) is unknown. The purpose of this study was to evaluate PK following LSG for two tacrolimus (FK) formulations (Astagraf XL® and Prograf®) with mycophenolate mofetil (MMF) in end stage renal disease (ESRD) patients awaiting renal transplant (RTx) across genotypes.
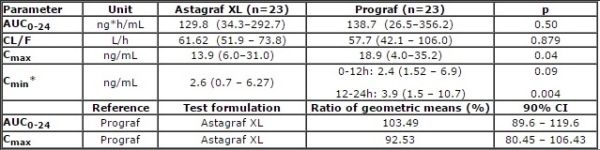
Methods: Open-label, randomized, two-way crossover PK study was conducted. RTx candidates who were >3 months post-LSG were randomized to Astagraf XL® 8mg (1 dose) or Prograf® 4mg (2 doses 12h apart) with MMF 1gm (2 doses 12h apart). 24-hour PK profiles were analyzed by LCMS and compared. Genotyping was performed for MDR1/ABCB1, CYP3A4, and CYP3A5. PK differences across genotypes were assessed with non-parametric statistics. Astagraf XL® bioequivalence to Prograf® was assessed by 90%CI for LSMeans ratio of AUC0-24 and Cmax. Results were compared to a historic control (Clin Transplant 2008: 22: 281–291).
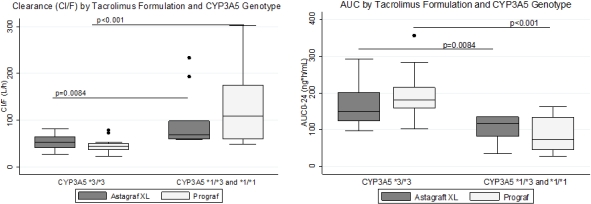
Results: 23 patients completed the study: male (56.5%), Caucasian (56.5%), mean age of 50.8 years (+11.4). Table 1 includes PK analysis and bioequivalence results. Figure 1 presents CYP3A5*1 conferred PK differences. CYP3A4*22 and ABCB1 did not impact FK PK. Ideal (R2=0.15) and actual (R2=0.229) body weights poorly correlated with FK AUC0-24. More tacrolimus variability was seen in patients with gastric bypass vs. LSG.
Conclusions: Single dose PK analysis revealed Astagraf XL® and Prograf® were bioequivalent. CYP3A5*1 allele influences PK parameters of both FK formulations in an LSG population. Alternative starting doses of Prograf® and Astagraf XL® following transplant are not necessary.
 .
.
CITATION INFORMATION: Lichvar A, Leino A, Kaiser T, Mizuno T, Fukuda T, Christians U, Alloway R, Vinks A, Woodle E, Diwan T. Pharmacokinetic Evaluation of Astagraf XL® and Prograf® in Renal Transplant Candidates Following Laparoscopic Sleeve Gastrectomy. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Lichvar A, Leino A, Kaiser T, Mizuno T, Fukuda T, Christians U, Alloway R, Vinks A, Woodle E, Diwan T. Pharmacokinetic Evaluation of Astagraf XL® and Prograf® in Renal Transplant Candidates Following Laparoscopic Sleeve Gastrectomy. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/pharmacokinetic-evaluation-of-astagraf-xl-and-prograf-in-renal-transplant-candidates-following-laparoscopic-sleeve-gastrectomy/. Accessed March 6, 2026.« Back to 2016 American Transplant Congress
