Pharmacokinetic Analysis of Direct Acting Antiviral Use on Weight-Adjusted FK506 Trough/dose Ratios in Obese Kidney Transplant Recipients
A. Demirag1, P. L. Lobo2, J. Oberholzer1, A. Kumar2, B. Rawashdeh1, S. L. Lennon1, H. N. Guvener Demirag1, A. Doyle2, J. Geystone3, K. L. Brayman1
1Transplantation, University of Virginia Medical Center, Charlottesville, VA, 2Transplantation Nephrology, University of Virginia Medical Center, Charlottesville, VA, 3Clinical Pharmacy, University of Virginia Medical Center, Charlottesville, VA
Meeting: 2021 American Transplant Congress
Abstract number: 156
Keywords: FK506, Hepatitis C, Kidney transplantation, Pharmacokinetics
Topic: Clinical Science » Pharmacy » Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Information
Session Name: The Metabolism Milleu: Updates in Pharmacokinetics and Pharmacogenomics
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 6, 2021
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:30pm-6:35pm
Presentation Time: 6:30pm-6:35pm
Location: Virtual
*Purpose: Using hepatitis C viremic (HCV) organs in kidney transplant recipients(KTRs) is very important to decrease the number of patients on the waiting list. Direct acting antivirals (DAA) are highly effective at curing HCV infection, but the pharmacokinetic implications of DAA use on calcineurin inhibitor therapy in KTRs are incompletely described. The aim of this study is to investigate the effects of DAA on FK506(FK) trough/dose (T/D) ratio and rejection rate in KTRs.
*Methods: This is a single-center, retrospective analysis of HCV negative KTRs who received HCV kidneys followed by 12-weeks of DAA therapy. Immunosuppression(IS) was administered based on our transplant center protocol: prednisone, FK and mycophenolate. Dose of FK was adjusted to keep FK levels at desired levels based on our IS protocol. FK T/D ratio was determined while patients were on a stable dose of FK to maintain the desired steady-state T level prior to, during, and after DAA treatment. FK T levels were quantitated twice per week and a steady state was determined when the desired level was attained at three consecutive measurements.
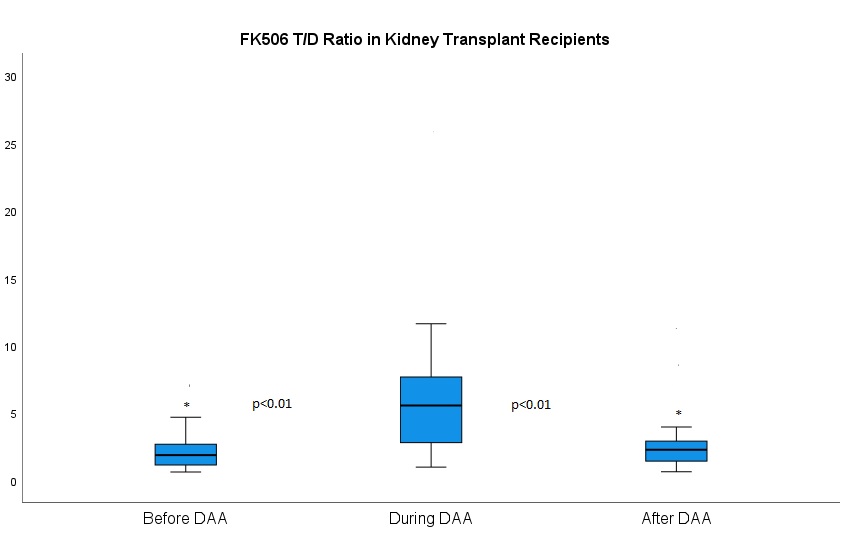
*Results: Fourty-four HCV negative organ recipients received HCV positive deceased donor kidney allografts between 3-2019 and 6-2020. Median age was 57 years (IQR 50-66). The FK T/D ratio was greater during DAA treatment (5.54, IQR 2.79-7.65) compared to before treatment (1.85, IQR 1.12-2.67) (p<0.01), after completion of treatment (2.25, IQR 1.41-2.89) (p<0.01). Six KTRs developed cellular acute rejection (ACR) post DAA treatment within the first 6 months post-transplant(p<0.01).
*Conclusions: DAA treatment in kidney transplant recipients decreases FK elimination leading to a decrease, by about 40% in FK dosing during DAA therapy. Cessation of DAA therapy leads to an increase in ACR possibly because of delays in increasing the FK dose. Larger studies are warranted to validate these findings.
To cite this abstract in AMA style:
Demirag A, Lobo PL, Oberholzer J, Kumar A, Rawashdeh B, Lennon SL, Demirag HNGuvener, Doyle A, Geystone J, Brayman KL. Pharmacokinetic Analysis of Direct Acting Antiviral Use on Weight-Adjusted FK506 Trough/dose Ratios in Obese Kidney Transplant Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/pharmacokinetic-analysis-of-direct-acting-antiviral-use-on-weight-adjusted-fk506-trough-dose-ratios-in-obese-kidney-transplant-recipients/. Accessed March 2, 2026.« Back to 2021 American Transplant Congress